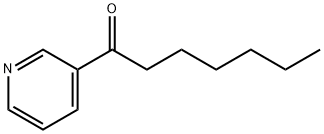
1-Pyridin-3-yl-heptan-1-one synthesis
- Product Name:1-Pyridin-3-yl-heptan-1-one
- CAS Number:6294-61-7
- Molecular formula:C12H17NO
- Molecular Weight:191.27

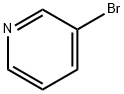
21369-64-2
137 suppliers
$73.20/100ml
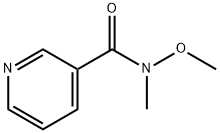
95091-91-1
41 suppliers
$60.00/50mg

6294-61-7
7 suppliers
inquiry
Yield:6294-61-7 78%
Reaction Conditions:
in tetrahydrofuran;hexane at -78; for 0.5 h;
Steps:
1 -Pyridin-3-yl-heptan-i -one. (compound 3, Scheme 1 ) ;
References:
WO2008/129054,2008,A2 Location in patent:Page/Page column 28-29
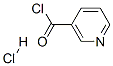
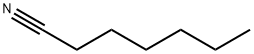
20260-53-1
199 suppliers
$16.00/5g

6294-61-7
7 suppliers
inquiry