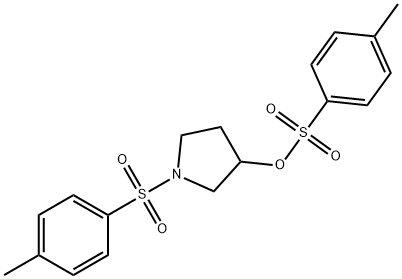
1-Tosyl-3-pyrrolidinol Tosylate synthesis
- Product Name:1-Tosyl-3-pyrrolidinol Tosylate
- CAS Number:131912-34-0
- Molecular formula:C18H21NO5S2
- Molecular Weight:395.49
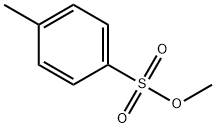
80-48-8
438 suppliers
$5.00/25g

170456-83-4
12 suppliers
$225.00/100mg

131912-34-0
9 suppliers
$262.00/100mg
Yield:-
Steps:
3 Preparation of 1-tosyl-3-(S)-(-)-tosyloxypyrrolidine STR17
PREPARATION 3 Preparation of 1-tosyl-3-(S)-(-)-tosyloxypyrrolidine STR17 Methyl para-toluenesulphonate (54 g) was added in portions to a solution of 1-tosyl-3-(R)-(-)-hydroxypyrrolidine (49 g--see Preparation 2) and triphenylphosphine (76 g) in anhydrous tetrahydrofuran (700 ml) at 0° C. The mixture was cooled to -20° C. and diethyl azodicarboxylate (58 g--"DEAD") was added, dropwise, over 30 minutes. During this time, the temperature of the mixture was not allowed to rise above -10° C. When the addition was complete the mixture was allowed to warm to room temperature and stirred for 16 hours. The mixture was concentrated in vacuo to give a solid which was purified by column chromatography on silica eluding with hexane containing dichloromethane (50%). The product-containing fractions were combined and concentrated in vacuo to give an oil which was crystallized from 1-propanol to give the title compound as a colourless solid, yield 56 g, m.p. 110° C., [α]D25 -5.2° (c 1.0, CH2 Cl2). Analysis %: Found: C,54.62; H,5.46; N,3.14. Calculated for C18 H21 NO5 S2: C,54.66; H,5.35; N,3.54. 1 H-N.M.R. (CDCl3) δ=7.75-7.65 (m, 4H); 7.40-7.30 (m, 4H); 5.00-4.90 (m, 1H); 3.55-3.35 (m, 3H); 3.30-3.20 (m, 1H); 2.50 (s, 3H); 2.45 (s, 3H); 2.10-1.90 (m, 2H) ppm.
References:
Pfizer Inc. US5281601, 1994, A

40499-83-0
204 suppliers
$14.00/1g

98-59-9
584 suppliers
$9.00/5g

131912-34-0
9 suppliers
$262.00/100mg