
1-Trimethylsilyl-1-butyne synthesis
- Product Name:1-Trimethylsilyl-1-butyne
- CAS Number:62108-37-6
- Molecular formula:C7H14Si
- Molecular Weight:126.27
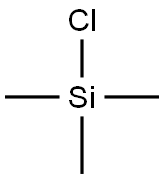
75-77-4
570 suppliers
$5.04/10
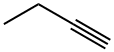
107-00-6
70 suppliers
$85.00/25 g

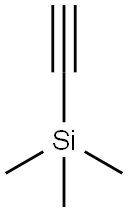
62108-37-6
52 suppliers
$11.00/100mg
Yield: 75%
Reaction Conditions:
Stage #1:but-1-yne with n-butyllithium in diethyl ether;hexane at -78; for 1.5 h;Inert atmosphere;
Stage #2:chloro-trimethyl-silane in diethyl ether;hexane at 20; for 12 h;Inert atmosphere;
Steps:
(Z)-1-(Trimethylsilyl)but-1-ene (2f)
BuLi (2.6 M in hexane, 194 mL, 504 mmol) was addedto a stirred solution of but-1-yne (28.0 g, 518 mmol) in Et2O (500mL) over 30 min at -78 °C. After 1 h chlorotrimethylsilane (64.0 mL, 504 mmol)was added to the lithium acetylide solution over 10 min. After 10 min thereaction mixture was warmed to room temperature and stirred for 12 h. Theresultant mixture was quenched with water and extractedwith Et2O. The combined organic layer was washed with brine, dried overNa2SO4, and concentrated by distillation at atmospheric pressure. Furtherdistillation of the residual oil at atmospheric pressure gave1-trimethylsilylbut-1-yne (47.6 g, 377 mmol, 75%). 1-Trimethylsilylbut-1- yne [62108-37-6]19: Bp 110-113 °C. 1HNMR (CDCl3) d 0.15 (s, 9H), 1.15 (t, J =7.6 Hz, 3H), 2.24 (q, J = 7.6 Hz,2H). Cyclohexene (44.6mL, 440 mmol) was slowly added to a stirred solution of BH3•SMe2(20.9 mL, 220 mmol) in THF (400 mL) at 0 °C. After 3 h 1-trimethylsilylbut-1-yne(27.6 g, 219 mmol) was added to the mixture over 10 min. After 15 min thereaction mixture was warmed to room temperature and stirred for 90 min. Thenthe resultant mixture was evaporated, diluted with pentane (100 mL), and cooledto 0 °C. AcOH (12.6 mL, 220 mmol) was added to the pentane solution. Afterbeing stirred for 90 min, the reaction mixture was distilled under reducedpressure to give a mixture of the title compound and pentane (30 °C, 13 Torr).Purification of the mixture by distillation at atmospheric pressure gave thetitle compound (20.6 g, 161 mmol, 73%). Bp110-115 °C. IR (neat) 2965, 1608, 1249, 838 cm-1; 1H NMR (CDCl3) d 0.12 (s, 9H), 0.99 (t, J =7.5 Hz, 3H), 2.14 (quint-d, J = 7.5,1.0 Hz, 2H), 5.45 (dt, J = 14.0, 1.0Hz, 1H), 6.30 (dt, J = 14.0, 7.4 Hz,1H); 13C NMR (CDCl3) d 0.2, 14.2, 26.8, 128.2, 150.7.
References:
Kinoshita, Hidenori;Kizu, Ryosuke;Inoue, Gen;Fujimoto, Masayuki;Saito, Masanori;Ichikawa, Junji;Hosomi, Akira;Miura, Katsukiyo [Tetrahedron Letters,2015,vol. 56,# 5,p. 713 - 716] Location in patent:supporting information

75-03-6
363 suppliers
$11.00/5g
1066-54-2
465 suppliers
$9.00/10g

62108-37-6
52 suppliers
$11.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
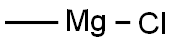
676-58-4
283 suppliers
$15.00/25ml

62108-37-6
52 suppliers
$11.00/100mg
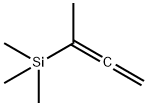
74542-82-8
12 suppliers
inquiry
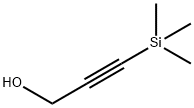
5272-36-6
191 suppliers
$6.00/1g

62108-37-6
52 suppliers
$11.00/100mg

74542-82-8
12 suppliers
inquiry