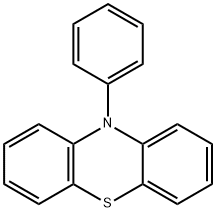
10-Phenyl-10H-phenothiazine synthesis
- Product Name:10-Phenyl-10H-phenothiazine
- CAS Number:7152-42-3
- Molecular formula:C18H13NS
- Molecular Weight:275.37

108-86-1
518 suppliers
$10.00/5g
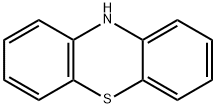
92-84-2
551 suppliers
$10.00/5g

7152-42-3
64 suppliers
$45.00/250mg
Yield:7152-42-3 97%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);potassium tert-butylate;tri tert-butylphosphoniumtetrafluoroborate in toluene for 20 h;Inert atmosphere;Reflux;
Steps:
General procedure A - synthesis of N-phenylphenothiazines.
General procedure: N-phenylphenothiazineswere synthesized similarily to the reported procedure [S2]. Phenothiazine(1.00 equiv) was dissolved in anhydrous toluene (0.51 M). Bromobenzene(1.22 equiv), KOt-Bu (1.29 equiv) and (t-Bu)3PHBF4 (6 mol %) were added, followedby Pd2dba3 (3 mol % as 6 mol % Pd). The reaction mixture was degassed usingthree freeze-pump-thaw cycles and finally stirred under reflux for 20 °h. Afterreaching room temperature, 100 mL EtOAc and 50 mL water were added to thereaction mixture. After phase separation, the aqueous phase was extractedadditionally with 3 × 100 mL EtOAc. The combined organic phases were dried overNa2SO4, the solvent evaporated and the crude product purified by columnchromatography.
References:
Speck, Fabienne;Rombach, David;Wagenknecht, Hans-Achim [Beilstein Journal of Organic Chemistry,2019,vol. 15,p. 52 - 59] Location in patent:supporting information

92-84-2
551 suppliers
$10.00/5g
591-50-4
498 suppliers
$10.00/1g

7152-42-3
64 suppliers
$45.00/250mg
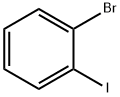
583-55-1
479 suppliers
$7.00/5g
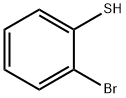
6320-02-1
149 suppliers
$14.00/1g

62-53-3
678 suppliers
$10.00/1g

7152-42-3
64 suppliers
$45.00/250mg

92-84-2
551 suppliers
$10.00/5g
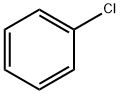
108-90-7
670 suppliers
$10.00/25G

7152-42-3
64 suppliers
$45.00/250mg