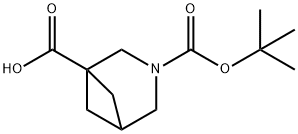
3-(TERT-BUTOXYCARBONYL)-3-AZABICYCLO[3.1.1]HEPTANE-1-CARBOXYLIC ACID synthesis
- Product Name:3-(TERT-BUTOXYCARBONYL)-3-AZABICYCLO[3.1.1]HEPTANE-1-CARBOXYLIC ACID
- CAS Number:1000931-22-5
- Molecular formula:C12H19NO4
- Molecular Weight:241.28
![3-Azabicyclo[3.1.1]heptane-3-carboxylic acid, 1-formyl-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1640998-93-1.gif)
1640998-93-1
2 suppliers
inquiry
![3-(TERT-BUTOXYCARBONYL)-3-AZABICYCLO[3.1.1]HEPTANE-1-CARBOXYLIC ACID](/CAS/20181022/GIF/1000931-22-5.gif)
1000931-22-5
20 suppliers
inquiry
Yield: 1.53 g
Reaction Conditions:
with sodium chlorite;disodium hydrogenphosphate in tetrahydrofuran;water;tert-butyl alcohol at 20; for 1 h;
Steps:
3-(tert-Butoxycarbonyl)-3-azabicyclo[3.1.1]heptane-1-carboxylic acid (24).
Alcohol 22 (2.27 g,10 mmol) and Dess-Martin periodinane (5.09 g, 12 mmol) were refluxed in CH2Cl2 (150 mL) for 3 h,and then stirred at rt for 12 h. The precipitate was filtered off, and the filtrate was evaporated in vacuo.The residue was subjected to flash chromatography (Hexanes - EtOAc (1 : 1) as eluent, Rf = 0.52) togive crude 23 (2.01 g), which was dissolved in THF (65 mL), and 2-methyl-2-butene (6.26 g, 89 mmol)in tert-butanol (65 mL) was added, followed by H2O (45 mL), NaClO2 (1.59 g, 18 mmol), and Na2HPO4(4.29 g, 36 mmol). The resulting mixture was stirred at rt for 1 d, then evaporated in vacuo, diluted withH2O (50 mL), and extracted with CH2Cl2 (350 mL). The combined extracts were dried over Na2SO4and evaporated in vacuo. The crude product was dissolved in 1 M aq NaOH (50 mL), washed withCH2Cl2 (350 mL). The aqueous phase was acidified with 1 M aq NaHSO4 to pH = 3-4 and extractedwith EtOAc (3100 mL). The combined extracts were dried over Na2SO4 and evaporated in vacuo togive 24 (1.53 g, 63% from 22). An analytical sample was obtained by recrystallization from benzene.White crystals. Mp 172-174 C. MS (m/z, ESI): 242 (MH+). Anal. calc. for C12H19NO4 C 59.73, H 7.94,N 5.80. Found C 59.48, H 8.27, N 5.53. 1H NMR (CDCl3) 9.38 (br s, 1H), 3.65 (s, 0.8H), 3.62 (s,1.2H), 3.47 (s, 1.2H), 3.44 (s, 0.8H), 2.32-2.41 (m, 3H), 1.53-1.55 (m, 2H), 1.42 (s, 9H). 13C NMR(CDCl3) 178.5 and 178.1 (C), 156.32 and 156.30 (C), 80.1 and 80.0 (C), 49.9 and 49.6 (CH2), 49.3 and48.8 (CH2), 44.1 (C), 34.33 and 34.28 (CH2), 28.7 (CH3), 28.6 (CH).
References:
Tymtsunik, Andriy V.;Bilenko, Vitaliy A.;Grygorenko, Oleksandr O.;Komarov, Igor V. [Synlett,2014,vol. 25,# 3,art. no. ST-2013-B0898-L,p. 355 - 358] Location in patent:supporting information
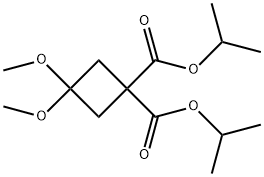
115118-68-8
143 suppliers
$13.00/1g
![3-(TERT-BUTOXYCARBONYL)-3-AZABICYCLO[3.1.1]HEPTANE-1-CARBOXYLIC ACID](/CAS/20181022/GIF/1000931-22-5.gif)
1000931-22-5
20 suppliers
inquiry
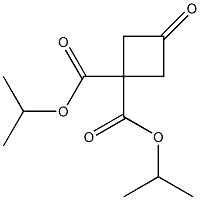
893724-10-2
56 suppliers
$10.00/1g
![3-(TERT-BUTOXYCARBONYL)-3-AZABICYCLO[3.1.1]HEPTANE-1-CARBOXYLIC ACID](/CAS/20181022/GIF/1000931-22-5.gif)
1000931-22-5
20 suppliers
inquiry
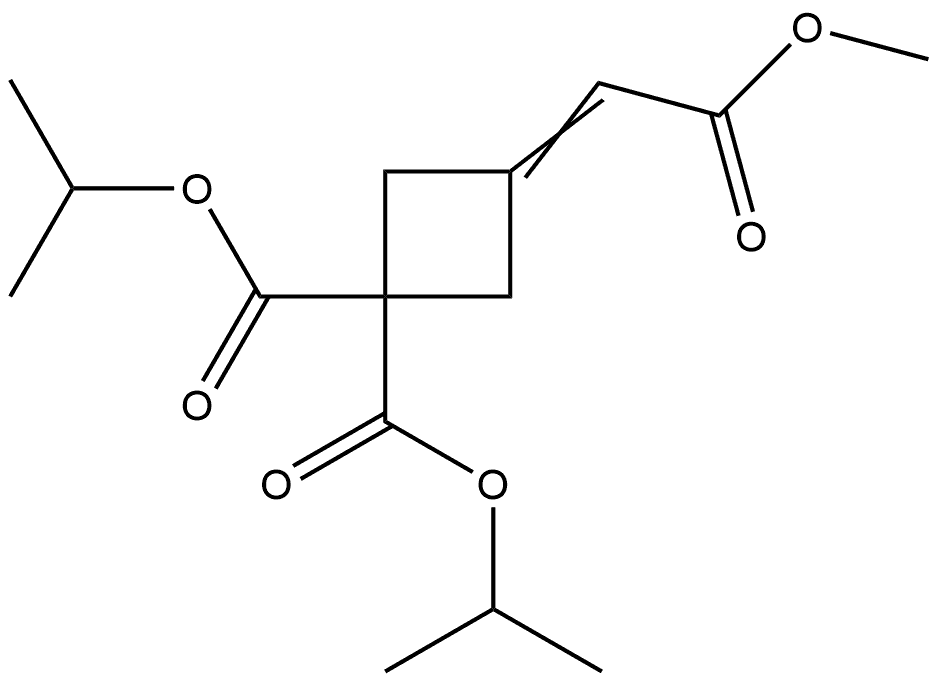
1628783-86-7
0 suppliers
inquiry
![3-(TERT-BUTOXYCARBONYL)-3-AZABICYCLO[3.1.1]HEPTANE-1-CARBOXYLIC ACID](/CAS/20181022/GIF/1000931-22-5.gif)
1000931-22-5
20 suppliers
inquiry
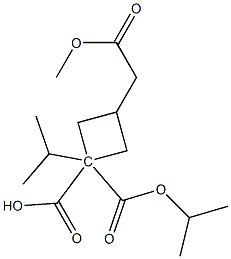
1628783-87-8
3 suppliers
inquiry
![3-(TERT-BUTOXYCARBONYL)-3-AZABICYCLO[3.1.1]HEPTANE-1-CARBOXYLIC ACID](/CAS/20181022/GIF/1000931-22-5.gif)
1000931-22-5
20 suppliers
inquiry