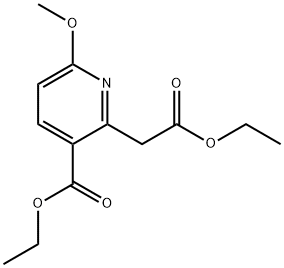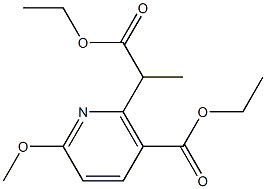
ethyl 2-(1-ethoxy-1-oxopropan-2-yl)-6-methoxynicotinate synthesis
- Product Name:ethyl 2-(1-ethoxy-1-oxopropan-2-yl)-6-methoxynicotinate
- CAS Number:1003589-78-3
- Molecular formula:C14H19NO5
- Molecular Weight:281.3044

74-88-4
341 suppliers
$15.00/10g

1003589-78-3
5 suppliers
inquiry
Yield:1003589-78-3 96%
Reaction Conditions:
Stage #1: ethyl 2-(2-ethoxy-2-oxoethyl)-6-methoxynicotinatewith sodium t-butanolate in tetrahydrofuran at 0; for 0.5 h;
Stage #2: methyl iodide in tetrahydrofuran at 0 - 20; for 2 h;
Stage #3: with acetic acid in tetrahydrofuran at 0; for 0.5 h;
Steps:
3.2
Example 3.2: Ethyl 2-(2-ethoxy-1-methyl-2-oxoethyl)-6- methoxynicotinate; To a suspension of sodium tert-butoxide (7.98 g, 82.3 mmol) in tetrahydrofuran (250 ml_) was added a solution of ethyl 2-(2-ethoxy-2- oxoethyl)-6-methoxynicotinate (20.0 g, 74.8 mmol) (the title compound from Example 3.1) in tetrahydrofuran (100 mL) at 0 0C over 15 minutes. After being stirred for 15 minutes, iodomethane (53.1 g, 374 mmol) was added at 0 0C. The reaction was allowed to warm up to r.t. over 2 hours. Acetic acid (1 mL) was added at 0 0C and the mixture was stirred for 30 minutes. The reaction was diluted with water and extracted with ethyl acetate. The organic layer was dried over magnesium sulfate, filtered and concentrated to give the title compound (20.16 g, 96%). 1H NMR (300 MHz, CDCl3): δ(ppm) B.16 (d,
References:
WO2008/9125,2008,A1 Location in patent:Page/Page column 56