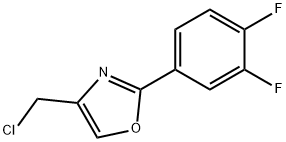
4-(chloromethyl)-2-(3,4-difluorophenyl)-1,3-oxazole synthesis
- Product Name:4-(chloromethyl)-2-(3,4-difluorophenyl)-1,3-oxazole
- CAS Number:1005336-42-4
- Molecular formula:C10H6ClF2NO
- Molecular Weight:229.61
Yield:1005336-42-4 47%
Reaction Conditions:
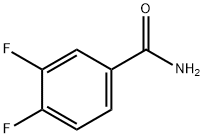
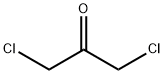
Stage #1: (3,4-difluoro-phenyl)-amide;1,3-Dichloroacetone at 135; for 1 h;
Stage #2: with sulfuric acid at 20; for 0.25 h;
Steps:
22A
Example 22A; 4-(Chloromethyl)-2-(3,4-difluorophenyl)-1,3-oxazole; With stirring, 500 mg (3.94 mmol) of 1,3-dichloroacetone and 619 mg (3.94 mmol) of 3,4-difluorobenzamide are heated at +135° C. for 1 h. After cooling to RT, the mixture is allowed to stand at this temperature for 90 min 1.0 ml of conc. sulphuric acid is then added, and the mixture is stirred at RT for a further 15 min. The entire mixture is then poured onto 50 ml of ice. Initially, a viscous oil is formed. The mixture is stirred for 60 min, during which time a precipitate is formed which is filtered off with suction, dried under high vacuum and then purified chromatographically on silica gel 60 (mobile phase: isohexane/ethyl acetate 10:1).Yield: 429 mg (47% of theory)1H-NMR (400 MHz, DMSO-d6): δ=8.31 (s, 1H), 8.02-7.93 (m, 1H), 7.88-7.81 (m, 1H), 7.68-7.59 (m, 1H), 4.75 (s, 2H).LC-MS (Method 2): Rt=2.40 min; MS (ESIpos): m/z=230 [M+H]+.
References:
US2010/93728,2010,A1 Location in patent:Page/Page column 21
34036-07-2
351 suppliers
$7.00/5g

1005336-42-4
5 suppliers
inquiry