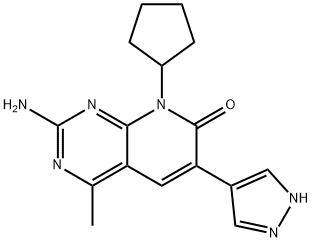
Pyrido[2,3-d]pyrimidin-7(8H)-one, 2-amino-8-cyclopentyl-4-methyl-6-(1H-pyrazol-4-yl)- synthesis
- Product Name:Pyrido[2,3-d]pyrimidin-7(8H)-one, 2-amino-8-cyclopentyl-4-methyl-6-(1H-pyrazol-4-yl)-
- CAS Number:1013098-90-2
- Molecular formula:C16H18N6O
- Molecular Weight:310.35
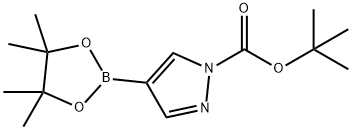
552846-17-0
225 suppliers
$11.00/1g
![2-amino-6-bromo-8-cyclopentyl-4-methylpyrido[2,3-d]pyrimidin-7(8H)-one](/CAS/20180601/GIF/934495-31-5.gif)
934495-31-5
1 suppliers
inquiry
![Pyrido[2,3-d]pyrimidin-7(8H)-one, 2-amino-8-cyclopentyl-4-methyl-6-(1H-pyrazol-4-yl)-](/CAS/20180601/GIF/1013098-90-2.gif)
1013098-90-2
5 suppliers
inquiry
Yield:1013098-90-2 65%
Reaction Conditions:
with potassium carbonate;bis-triphenylphosphine-palladium(II) chloride in N,N-dimethyl-formamide at 120; for 0.166667 h;Microwave irradiation;
Steps:
5
Example 5. Preparation of 2-amino-8-cvclopentyl-6-(1 H-pyrazol-4-yl)-4-methylpyridof2,3-Qipyrimidin- 7(8H)-one (Compound 101 );
References:
WO2008/32162,2008,A1 Location in patent:Page/Page column 36-37
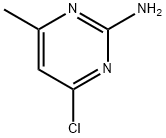
5600-21-5
319 suppliers
$18.00/25g
![Pyrido[2,3-d]pyrimidin-7(8H)-one, 2-amino-8-cyclopentyl-4-methyl-6-(1H-pyrazol-4-yl)-](/CAS/20180601/GIF/1013098-90-2.gif)
1013098-90-2
5 suppliers
inquiry
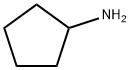
1003-03-8
211 suppliers
$10.00/1g
![Pyrido[2,3-d]pyrimidin-7(8H)-one, 2-amino-8-cyclopentyl-4-methyl-6-(1H-pyrazol-4-yl)-](/CAS/20180601/GIF/1013098-90-2.gif)
1013098-90-2
5 suppliers
inquiry