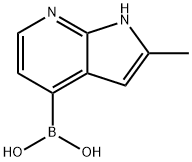
(2-METHYL-1H-PYRROLO[2,3-B]PYRIDIN-4-YL)BORONIC ACID synthesis
- Product Name:(2-METHYL-1H-PYRROLO[2,3-B]PYRIDIN-4-YL)BORONIC ACID
- CAS Number:1014614-07-3
- Molecular formula:C8H9BN2O2
- Molecular Weight:175.98

5419-55-6
373 suppliers
$12.00/5g
![4-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/1014613-64-9.gif)
1014613-64-9
67 suppliers
$45.00/100mg
![(2-METHYL-1H-PYRROLO[2,3-B]PYRIDIN-4-YL)BORONIC ACID](/CAS/20180629/GIF/1014614-07-3.gif)
1014614-07-3
14 suppliers
inquiry
Yield:1014614-07-3 41%
Reaction Conditions:
Stage #1: 4-bromo-2-methyl-1H-pyrrolo[2,3-b]pyridinewith sodium hydride in tetrahydrofuran;mineral oil at 0;Inert atmosphere;
Stage #2: with n-butyllithium in tetrahydrofuran;hexanes;mineral oil at -78;Inert atmosphere;
Stage #3: Triisopropyl borate
Steps:
11
Description 11 (2-methyl-1W-pyrrolo[2,3-b]pyridin-4-yl)boronic acidH To a stirred suspension of sodium hydride 60% dispersion on mineral oil (380 mg, 9.48 mmol) in tetrahydrofuran (8 ml_) at 00C under nitrogen was added a solution of 4-bromo-2- methyl-1 H-pyrrolo[2,3-]pyridine (D10) (1.6 g, 7.58 mmol) in tetrahydrofuran (24 ml.) dropwise. The reaction was degassed and cooled to -78°C and to the reaction was added n-butyllithium 2.5M in hexanes (6 ml_, 14.96 mmol) dropwise. The reaction was stirred at - 78°C for 30 mins. To the reaction was added triisopropylborate (5.7 ml_, 22.74 mmol) and stirring at -78°C continued for 1 h. The reaction was allowed to warm to 00C and was treated with water (50 ml_) the organic layer was separated and the aqueous extracted with ethylacetate (2 x 40 ml_) the combined organics were washed with 2M aqueous sodium hydroxide (50 ml_). The aqueous phases were combined and the pH adjusted to pH = 7 using 2M aqueous hydrochloric acid. The neutral aqueous was extracted with ethylacetate (2 x 50 ml_). The extracts were concentrated in vacuo to yield the title compound as a cream solid (550 mg, 41 %). LC/MS (Method A): MH+ = 177 seen at retention time 1.73 mins.
References:
WO2009/112475,2009,A1 Location in patent:Page/Page column 63