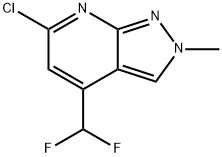
6-chloro-4-(difluoromethyl)-2-methyl-2H-pyrazolo[3,4-b]pyridine synthesis
- Product Name:6-chloro-4-(difluoromethyl)-2-methyl-2H-pyrazolo[3,4-b]pyridine
- CAS Number:1018165-42-8
- Molecular formula:C8H6ClF2N3
- Molecular Weight:217.6
Yield:1018165-42-8 0.145 g
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 20 h;Inert atmosphere;
Steps:
11 Example 11 : (compound 106) 2-Amino-5-[4-(difluoromethyl)-2-methyl-2H-pyrazolo[3,4-fe]pyridin-6-yl]benzonitrile 6-chloro-4-(difluoromethyl)-2-methyl-2H-pyrazolo[3,4-fe]pyridine
To 1 g (4.91 mmol) of 6-chloro-4-(difluoromethyl)-1 H-pyrazolo[3,4-b]pyridine in 20 ml of anhydrous DMF, under an inert atmosphere of nitrogen, are added 0.37 ml (5.89 mmol) of methyl iodide and 0.814 g (5.89 mmol) of potassium carbonate, at room temperature. The reaction medium is stirred for 20 hours and then hydrolysed with water. The aqueous phase is extracted with ethyl acetate. The organic phase obtained is washed with water, dried over sodium sulfate and then concentrated under reduced pressure. The colourless oil obtained is purified by column chromatography on silica gel, eluting with a heptane/dichloromethane mixture. 0.145 g of a white solid are obtained. MH+: 218 Melting point: 152°C
References:
WO2013/87744,2013,A1 Location in patent:Page/Page column 33
![1H-Pyrazolo[3,4-b]pyridine, 6-chloro-4-(difluoromethyl)-](/CAS/20210305/GIF/1279829-79-6.gif)
