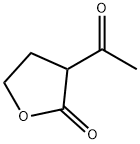
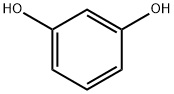
7-HYDROXY-3-(2-HYDROXYETHYL)-4-METHYL-2H-CHROMEN-2-ONE synthesis
- Product Name:7-HYDROXY-3-(2-HYDROXYETHYL)-4-METHYL-2H-CHROMEN-2-ONE
- CAS Number:10185-02-1
- Molecular formula:C12H12O4
- Molecular Weight:220.22
Yield:10185-02-1 88%
Reaction Conditions:
with magnetic nanoparticles functionalized ethane sulfonic acid at 90; for 3 h;Pechmann Condensation;
Steps:
General procedure for Pechmann reaction in the presence of MNESA catalyst
General procedure: A mixture of phenol (1.0 mmol), β-ketoester (1.5 mmol) and MNESA (0.075 g) was stirred at 90 °C in a round-bottomed flask for the appreciated time. After completion of the reaction as confirmed by TLC, the reaction mixture was cooled down to room temperature and the catalyst was separated from the reaction mixture using an external magnetic Some field. water was then added to the reaction mixture and the product was extracted using EtOAc (2 9 10 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated under vacuumto yield the crude product. For more purification, the crude product was purified by recrystallization in ethanol to obtain the desired purity.
References:
Samadizadeh, Marjaneh;Nouri, Saeed;Kiani Moghadam, Faeze [Research on Chemical Intermediates,2016,vol. 42,# 6,p. 6089 - 6103]