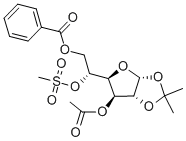
3-O-ACETYL-6-O -BENZOYL-5-O-(METHYLSULFONYL)-1,2-O-ISOPROPYLIDENE-ALPHA-D-GLUCOFURANOSE synthesis
- Product Name:3-O-ACETYL-6-O -BENZOYL-5-O-(METHYLSULFONYL)-1,2-O-ISOPROPYLIDENE-ALPHA-D-GLUCOFURANOSE
- CAS Number:102029-58-3
- Molecular formula:C19H24O10S
- Molecular Weight:444.45
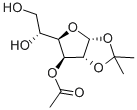
24807-96-3
24 suppliers
$80.00/500mg

98-88-4
577 suppliers
$10.00/5g
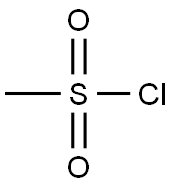
124-63-0
6 suppliers
$10.00/5g

102029-58-3
14 suppliers
inquiry
Yield:102029-58-3 90.6%
Reaction Conditions:
Stage #1: 3-O-acetyl-1,2-O-isopropylidene-α-D-glucofuranose;benzoyl chloridewith pyridine in tetrahydrofuran at 0 - 20; for 4 h;
Stage #2: methanesulfonyl chloridewith triethylamine in tetrahydrofuran at 0 - 20; for 2.5 h;
Steps:
3.c
c. Dissolve the substance obtained in step b (compound 4) in anhydrous tetrahydrofuran (1.2L) and add anhydrous pyridine (1.5mol),The solution was cooled to 0 with ice water and then benzoyl chloride BzCl (1.2mol) was added dropwise,After the dropwise addition is completed, react for 4h at room temperature. After the reaction is completed, continue to add triethylamine (2.5mol),After cooling to 0°C, methanesulfonyl chloride MsCl (92.89mL, 1.2mol) was added dropwise to the reaction solution. After the addition, the reaction was stirred at room temperature for 2.5h.After the reaction is completed, the reaction solution is slowly poured into a saturated sodium bicarbonate solution at 0°C,Separate the liquids and extract with dichloromethane. The extract phase is washed with water, 1.5mol/L hydrochloric acid aqueous solution, water, saturated brine, and then dried with anhydrous sodium sulfate.Filtration, concentration, and column chromatography under reduced pressure (eluent: petroleum ether: ethyl acetate = 7:1 (volume ratio)) to obtain 402.2 g of a white solid product (yield: 90.6%).
References:
CN113416220,2021,A Location in patent:Paragraph 0074-0076; 0079
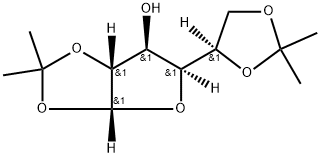
582-52-5
469 suppliers
$5.00/5g

102029-58-3
14 suppliers
inquiry
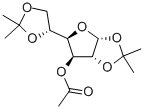
16713-80-7
56 suppliers
$55.00/5G

98-88-4
577 suppliers
$10.00/5g
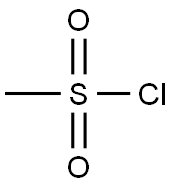
124-63-0
6 suppliers
$10.00/5g

102029-58-3
14 suppliers
inquiry