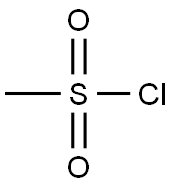n-Pentylmesylate, 98 % synthesis
- Product Name:n-Pentylmesylate, 98 %
- CAS Number:6968-20-3
- Molecular formula:C6H14O3S
- Molecular Weight:166.24
Yield: 98%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20;
Steps:
4.2.4. General procedures for the preparation of methanesulfonate 4a-c
Alcohols (50 mmol, 1 equiv.) and 11.08 mL Et3N (80 mmol, 1.6 equiv.) was dissolved in 60 mL CH2Cl2, and the solution was cooleddown to 0 °C, then 4.33 mL methanesulfonyl chloride (56 mmol, 1.12 equiv.) was introduced by syringe successively. The mixture is stirredfor 30 min at 0 °C, and then stirred overnight at room temperature. The organic layer is washed successively with 1M hydrochloric acid solution, saturated aqueous sodium bicarbonate solution, and brine. The organic layer is dried over Na2SO4, filtered, and concentrated under reduced pressure. The product was purified by column chromatography with silica gel (EtOAc: petroleum=1: 2). Pentyl methanesulfonate (4a) was obtained in 98% yield as a colorless liquid: 1H NMR (400 MHz, CDCl3) δ 4.20 (t, J=6.6 Hz, 2 H), 2.98(s, 3 H), 1.81-1.65 (m, 2 H), 1.46-1.23 (m, 4 H), 0.90 (t, J=7.0 Hz,3 H). LRMS (EI) m/z (%): 70 (100), 97 (79.58), 137 (2.04).
References:
Lin, Chao;Pan, Renming;Xing, Ping;Jiang, Biao [Journal of Fluorine Chemistry,2018,vol. 214,p. 35 - 41]