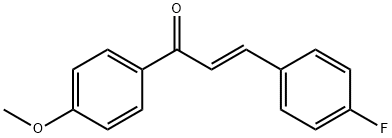
3-(4-fluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one synthesis
- Product Name:3-(4-fluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one
- CAS Number:102692-37-5
- Molecular formula:C16H13FO2
- Molecular Weight:256.27

459-57-4
584 suppliers
$6.00/25g
123-11-5
863 suppliers
$10.00/10g

102692-37-5
9 suppliers
$45.00/10mg
Yield:102692-37-5 91%
Reaction Conditions:
with lithium hydroxide monohydrate in ethanol at 20; for 4 h;Claisen-Schmidt Condensation;
Steps:
Preparation of chalcones via Claisen-Schmidt condensation
General procedure: A solution of the acetophenone(10 mmol) and LiOH.H2O (10 mol%) in 10 mL of absoluteethanol is stirred at the appropriate temperature for 10 min (for reactions at40 °C, the bulb is equipped with a reflux condenser). Then, the benzaldehyde(10 mmol, 1 equiv.) is added and the system is protected from theatmosphere with a cork stopper. Reaction progress is monitored by TLC orLCMS-analysis; during the course of the reaction, the chalcone may precipitate.Upon attaining maximum conversion grade, the reaction mixture is quenched with15 mL of 1% hydrochloric acid.If the chalcone has precipitated, it isisolated by means of filtration. In order to remove residual amounts ofbenzaldehyde, the residue is washed thoroughly with water until the filtrateturns clear. The obtained solid is the chalcone, which can be dried in adessicator. Subsequently, the chalcone can be recrystallized in absoluteethanol so as to obtain high purity crystals.If the chalcone has formed a separate oilyliquor at the bottom of the bulb, it can be extracted from the mixture withdiethyl ether. The organic phase is subsequently washed with brine (2x) anddried over MgSO4, upon which the solution is concentrated in vacuo. Again, purification of the thusobtained residue can be performed through recrystallization in absoluteethanol.Detailed reaction conditions and yields for chalcones 35-50are provided in Table S13
References:
Roman, Bart I.;De Ryck, Tine;Patronov, Atanas;Slavov, Svetoslav H.;Vanhoecke, Barbara W.A.;Katritzky, Alan R.;Bracke, Marc E.;Stevens, Christian V. [European Journal of Medicinal Chemistry,2015,vol. 101,art. no. 7956,p. 627 - 639] Location in patent:supporting information

459-57-4
584 suppliers
$6.00/25g
100-06-1
589 suppliers
$18.19/25g

102692-37-5
9 suppliers
$45.00/10mg
![1-[(4-Fluorophenyl)methyl]-1H-1,2,3-benzotriazole](/CAS/20210305/GIF/148004-76-6.gif)
148004-76-6
3 suppliers
inquiry

102692-37-5
9 suppliers
$45.00/10mg
![1-[1-(4-Fluorophenyl)-2-(trimethylsilyl)ethyl]-1H-1,2,3-benzotriazole](/CAS/20210305/GIF/196861-98-0.gif)
196861-98-0
3 suppliers
inquiry

102692-37-5
9 suppliers
$45.00/10mg