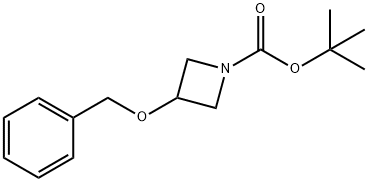
3-Benzyloxy-azetidine-1-carboxylicacidtert-butylester synthesis
- Product Name:3-Benzyloxy-azetidine-1-carboxylicacidtert-butylester
- CAS Number:1027995-71-6
- Molecular formula:C15H21NO3
- Molecular Weight:263.33

100-39-0
437 suppliers
$10.00/10g
141699-55-0
397 suppliers
$6.00/1g

1027995-71-6
32 suppliers
$495.11/5MG
Yield:1027995-71-6 89.9%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;mineral oil at 20; for 16 h;
Steps:
50.1 Step 1:
tert-butyl 3-(benzyloxy)azetidine-1-carboxylate
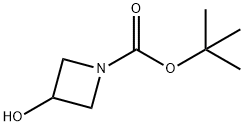
A mixture of tert-butyl 3-hydroxyazetidine-1-carboxylate (3.0 g, 17.32 mmol), benzyl bromide (3.55 g, 20.78 mmol) and sodium hydride (60% in mineral oil, 1.04 g, 25.98 mmol) in tetrahydrofuran (30 mL) was stirred at room temperature for 16 h.
The reaction was quenched with water (20 ml), and ethyl acetate (50 mL*3) was added for extraction.
The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated.
The residue was purified by flash chromatography (ethyl acetate/petroleum ether=0-30%) to give tert-butyl 3-(benzyloxy)azetidine-1-carboxylate 0135A (4.1 g, 89.9%) in the form of a colorless oil. LCMS (ESI) [M-56+H]+=208.2; [M+Na]+=286.21.837 min (Method A).
References:
US2020/369676,2020,A1 Location in patent:Paragraph 1144-1145

100-44-7
654 suppliers
$13.50/250G

141699-55-0
397 suppliers
$6.00/1g

1027995-71-6
32 suppliers
$495.11/5MG

24424-99-5
850 suppliers
$13.50/25G

1027995-71-6
32 suppliers
$495.11/5MG
398489-26-4
435 suppliers
$9.00/5g

1027995-71-6
32 suppliers
$495.11/5MG