
2-Thiophenecarboxylic acid, 5-chloro-4-(4-chloro-1-methyl-1H-pyrazol-5-yl)- synthesis
- Product Name:2-Thiophenecarboxylic acid, 5-chloro-4-(4-chloro-1-methyl-1H-pyrazol-5-yl)-
- CAS Number:1047630-61-4
- Molecular formula:C9H6Cl2N2O2S
- Molecular Weight:277.13

1047630-52-3
18 suppliers
inquiry

1047630-61-4
31 suppliers
inquiry
Yield:1047630-61-4 48%
Reaction Conditions:
Stage #1: methyl 5-chloro-4-(1-methylpyrazol-5-yl)-2-thiophenecarboxylatewith N-chloro-succinimide in tetrahydrofuran at 70; for 4 h;
Stage #2: with sodium hydroxide in tetrahydrofuran;water; for 2 h;
Stage #3: with hydrogenchloride in water;
Steps:
96.a
To a 500 ml. flask was added methyl 5-chloro-4-(1 -methyl-1 H-pyrazol-5-yl)-2-thiophenecarboxylate (5 g, 19.48 mmol) [from Example 95] and NCS (3.12 g, 23.37 mmol) in tetrahydrofuran (THF) (100 ml) (50 ml_). After stirring for 4 h at 70 0C, the yellow reaction solution was treated with 6M NaOH (32 ml_, 195 mmole) and stirred an additional 2 hours. The reaction solution was diluted with H2O (50 ml.)
References:
WO2008/98104,2008,A1 Location in patent:Page/Page column 190

24065-33-6
738 suppliers
$6.00/5g

1047630-61-4
31 suppliers
inquiry
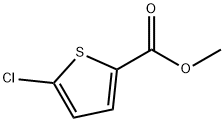
35475-03-7
114 suppliers
$17.00/1g

1047630-61-4
31 suppliers
inquiry
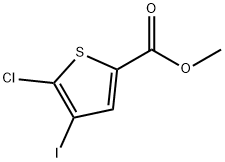
1047644-88-1
2 suppliers
inquiry

1047630-61-4
31 suppliers
inquiry
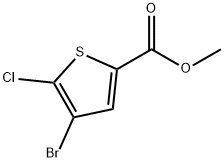
1047630-72-7
60 suppliers
$9.00/250mg

1047630-61-4
31 suppliers
inquiry