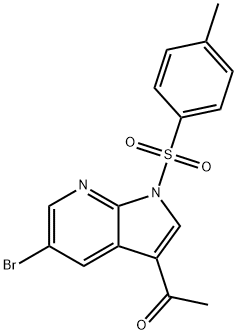
Ethanone, 1-[5-broMo-1-[(4-Methylphenyl)sulfonyl]-1H-pyrrolo[2,3-b]pyridin-3-yl]- synthesis
- Product Name:Ethanone, 1-[5-broMo-1-[(4-Methylphenyl)sulfonyl]-1H-pyrrolo[2,3-b]pyridin-3-yl]-
- CAS Number:1052633-38-1
- Molecular formula:C16H13BrN2O3S
- Molecular Weight:393.26

866545-96-2
52 suppliers
$29.00/100mg

98-59-9
584 suppliers
$9.00/5g
![Ethanone, 1-[5-broMo-1-[(4-Methylphenyl)sulfonyl]-1H-pyrrolo[2,3-b]pyridin-3-yl]-](/CAS/GIF/1052633-38-1.gif)
1052633-38-1
15 suppliers
$175.00/50mg
Yield:1052633-38-1 78.16%
Reaction Conditions:
Stage #1: 1-(5-bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)ethan-1-onewith sodium hydride in tetrahydrofuran; for 1 h;Cooling with ice;
Stage #2: 4-methylbenzene-1-sulfonyl chloride in tetrahydrofuran at 25; for 32 h;
Steps:
198.2 Step 2:
To a solution of 1-(5-bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)ethanone (7 g, 29.28 mmol) in tetrahydrofuran (60 mL), add sodium under ice-cooling Hydrogen (1.29 g, 32.21 mmol, 60% pure), after 1 hour, a solution of p-toluenesulfonyl chloride (6.70 g, 35.14 mmol) in tetrahydrofuran (4 mL) was added dropwise and the mixture was stirred at 25°C for 32 hours.The completion of the reaction was monitored by LCMS, the mixture was extracted with ethyl acetate and water, dried over sodium sulfate and concentrated by filtration, the residue was slurried with petroleum ether/ethyl acetate (5/1) to give 1-[5-bromo-1-( p-Tosyl)pyrrolo[2,3-b]pyridin-3-yl]ethanone (9.00 g, 78.16% yield) as a yellow solid.
References:
WO2022/184152,2022,A1 Location in patent:Page/Page column 193-194

98-59-9
584 suppliers
$9.00/5g
![Ethanone, 1-[5-broMo-1-[(4-Methylphenyl)sulfonyl]-1H-pyrrolo[2,3-b]pyridin-3-yl]-](/CAS/GIF/1052633-38-1.gif)
1052633-38-1
15 suppliers
$175.00/50mg
183208-35-7
534 suppliers
$5.00/1g
![Ethanone, 1-[5-broMo-1-[(4-Methylphenyl)sulfonyl]-1H-pyrrolo[2,3-b]pyridin-3-yl]-](/CAS/GIF/1052633-38-1.gif)
1052633-38-1
15 suppliers
$175.00/50mg