
TERT-BUTYL 8-BROMO-2,3-DIHYDROBENZO[F][1,4]OXAZEPINE-4(5H)-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 8-BROMO-2,3-DIHYDROBENZO[F][1,4]OXAZEPINE-4(5H)-CARBOXYLATE
- CAS Number:1055880-16-4
- Molecular formula:C14H18BrNO3
- Molecular Weight:328.2016
![Carbamic acid, N-[(4-bromo-2-hydroxyphenyl)methyl]-N-(2-hydroxyethyl)-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1445903-00-3.gif)
1445903-00-3
1 suppliers
inquiry
![TERT-BUTYL 8-BROMO-2,3-DIHYDROBENZO[F][1,4]OXAZEPINE-4(5H)-CARBOXYLATE](/CAS/20180529/GIF/1055880-16-4.gif)
1055880-16-4
5 suppliers
inquiry
Yield:1055880-16-4 86%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in dichloromethane at 20; for 12 h;
Steps:
11.2 Step 2: ferf-Butyl 8-bromo-2.3-dihydrobenzo[ ][1.4]oxazepine-4(5H)-carboxylate
Step 2: ferf-Butyl 8-bromo-2.3-dihydrobenzo[ ][1.4]oxazepine-4(5H)-carboxylate [01] To a solution of teri-butyl N-[(4-bromo-2-hydroxy-phenyl)methyl]-N-(2- hydroxyethyl)carbamate (3 g, 8.67 mmol) and triphenylphosphine (3.4 g, 13.0 mmol) in dichloromethane (85 mL) was added diisopropyl azodicarboxylate (2.69 g, 13.0 mmol) and the reaction was stirred at ambient temperature for 12 hours. The reaction was then washed with water, brine, dried with MgS04 and purified by silica gel column chromatography (0-40% EtOAc in heptane) to give teri-butyl 8-bromo-2,3- dihydrobenzo[ ][l,4]oxazepine-4(5H)-carboxylate (2.45 g, 86% yield). LCMS (m/z) ES+ 328 [M+l]+.
References:
WO2013/92941,2013,A1 Location in patent:Page/Page column 55

24424-99-5
824 suppliers
$13.50/25G
![8-BroMo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepine hydrochloride](/CAS/20130318/GIF/CB92645433.gif)
1352707-91-5
11 suppliers
inquiry
![TERT-BUTYL 8-BROMO-2,3-DIHYDROBENZO[F][1,4]OXAZEPINE-4(5H)-CARBOXYLATE](/CAS/20180529/GIF/1055880-16-4.gif)
1055880-16-4
5 suppliers
inquiry
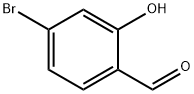
22532-62-3
309 suppliers
$5.00/250mg
![TERT-BUTYL 8-BROMO-2,3-DIHYDROBENZO[F][1,4]OXAZEPINE-4(5H)-CARBOXYLATE](/CAS/20180529/GIF/1055880-16-4.gif)
1055880-16-4
5 suppliers
inquiry