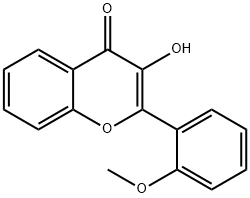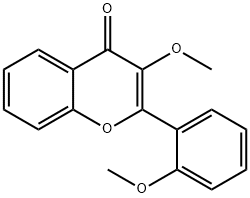
3-METHOXY-2-(2-METHOXYPHENYL)- 4H-1-BENZOPYRAN-4-ONE synthesis
- Product Name:3-METHOXY-2-(2-METHOXYPHENYL)- 4H-1-BENZOPYRAN-4-ONE
- CAS Number:105827-35-8
- Molecular formula:C17H14O4
- Molecular Weight:282.29
Yield: 74%
Reaction Conditions:
with potassium carbonate in acetonitrile at 40;
Steps:
6.1.3 General method for the preparation of 3-methoxy-flavones
General procedure: K2CO3 (1.8equiv.) and iodomethane (1.2equiv.) were added to a solution of flavonol (0.10-0.20mmol) at 40°C in dry CH3CN (1-2mL) until the starting material was totally consumed (as evidenced by TLC). The reaction mixture was then, partitioned between ethyl acetate and water. The ethyl acetate layer was washed with brine, dried over MgSO4, filtered and concentrated. The crude material was purified by column chromatography to yield methyl ethers.
References:
Burmistrova, Olga;Marrero, María Teresa;Estévez, Sara;Welsch, Isabel;Brouard, Ignacio;Quintana, José;Estévez, Francisco [European Journal of Medicinal Chemistry,2014,vol. 84,p. 30 - 41]

42220-77-9
55 suppliers
$57.50/1 g

105827-35-8
7 suppliers
inquiry
118-93-4
548 suppliers
$10.00/25g

105827-35-8
7 suppliers
inquiry
135-02-4
384 suppliers
$10.00/10g

105827-35-8
7 suppliers
inquiry