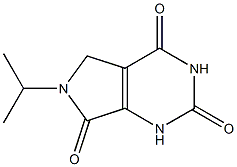
6-ISOPROPYL-5,6-DIHYDRO-1H-PYRROLO[3,4-D]PYRIMIDINE-2,4,7(3H)-TRIONE synthesis
- Product Name:6-ISOPROPYL-5,6-DIHYDRO-1H-PYRROLO[3,4-D]PYRIMIDINE-2,4,7(3H)-TRIONE
- CAS Number:1079649-91-4
- Molecular formula:C9H11N3O3
- Molecular Weight:209.2019

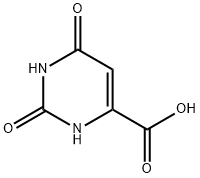
50-00-0
846 suppliers
$10.00/25g
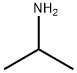
65-86-1
412 suppliers
$5.00/100mg
75-31-0
410 suppliers
$14.00/25mL
![6-ISOPROPYL-5,6-DIHYDRO-1H-PYRROLO[3,4-D]PYRIMIDINE-2,4,7(3H)-TRIONE](/CAS/20180601/GIF/1079649-91-4.gif)
1079649-91-4
6 suppliers
inquiry
Yield:1079649-91-4 73%
Reaction Conditions:
Stage #1: formaldehyd;orotic acid;isopropylamine in ethanol;water; for 16 h;Heating / reflux;
Stage #2: with hydrogenchloride in 2-methoxy-ethanol;water; for 16 h;Product distribution / selectivity;Heating / reflux;
Steps:
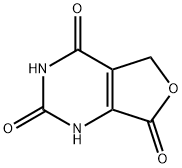
2
Alternatively, 2,4-Dihydroxy-6-isopropyl-5,6-dihydropyrrolo[3,4-d]pyrimidine-7-one (Intermediate 2) can be prepared in large scale following a procedure as shown below:Denatured ethanol (4 L) and 37% formaldehyde (551 mL, 7.4 mol) were added to orotic acid monohydrate (258 g, 1.48 mol). Isopropylamine (630 mL, 7.4 mol) was then slowly added to the suspension with stirring. The addition was slightly exothermic. The mixture was refluxed for 16 h. The reaction mixture was cooled in an ice bath, the white solid was collected by filtration and washed with ethanol to give the crude intermediate (409 g) as a solid. 1H NMR (300 MHz, DMSO-J6) δ ppm 1.20 (d, J= 6.5 Hz, 6H), 3.24 (qu, J= 6.6 Hz, IH), 3.90 (s, 2H). To the above crude intermediate (409 g) was added 2-methoxyethanol (1.9 L) and 12 N hydrochloric acid (190 mL). The reaction mixture was refluxed for 16 h, cooled in an ice bath and the solid was collected by filtration and washed with ethanol to give the title compound (228 g, 73 % over two steps) as a solid. 1U NMR (300 MHz, DMSO-J6) δ ppm 1.17 (d, J= 6.8 Hz, 6H), 4.12 (s, 2H), 4.24 (qu, J= 6.7 Hz, IH), 11.24 (s, IH), 11.79 (s, IH).
References:
WO2008/136756,2008,A1 Location in patent:Page/Page column 111
4156-75-6
35 suppliers
$45.00/10mg
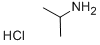
15572-56-2
132 suppliers
$14.14/1gm:
![6-ISOPROPYL-5,6-DIHYDRO-1H-PYRROLO[3,4-D]PYRIMIDINE-2,4,7(3H)-TRIONE](/CAS/20180601/GIF/1079649-91-4.gif)
1079649-91-4
6 suppliers
inquiry

50-00-0
846 suppliers
$10.00/25g
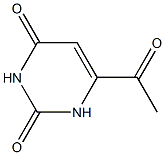
6341-93-1
2 suppliers
inquiry

75-31-0
410 suppliers
$14.00/25mL
![6-ISOPROPYL-5,6-DIHYDRO-1H-PYRROLO[3,4-D]PYRIMIDINE-2,4,7(3H)-TRIONE](/CAS/20180601/GIF/1079649-91-4.gif)
1079649-91-4
6 suppliers
inquiry