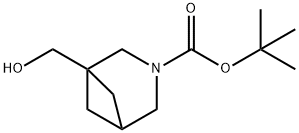
tert-butyl 1-(hydroxymethyl)-3-azabicyclo[3.1.1]heptane-3-carboxylate synthesis
- Product Name:tert-butyl 1-(hydroxymethyl)-3-azabicyclo[3.1.1]heptane-3-carboxylate
- CAS Number:1087784-78-8
- Molecular formula:C12H21NO3
- Molecular Weight:227.3

24424-99-5
823 suppliers
$13.50/25G
![{3-azabicyclo[3.1.1]heptan-1-yl}methanol](/CAS/20200331/GIF/1172693-01-4.gif)
1172693-01-4
1 suppliers
inquiry
![tert-butyl 1-(hydroxymethyl)-3-azabicyclo[3.1.1]heptane-3-carboxylate](/CAS/20181022/GIF/1087784-78-8.gif)
1087784-78-8
19 suppliers
inquiry
Yield:1087784-78-8 67%
Reaction Conditions:
in dichloromethane at 20; for 1.5 h;
Steps:
tert-Butyl 1-(hydroxymethyl)-3-azabicyclo[3.1.1]heptane-3-carboxylate (22)
To a solution of 21(2.37 g, 14 mmol) in THF (140 mL), BH3Me2S (10 M in THF, 7 mL, 70 mmol) was added dropwiseupon stirring. The resulting mixture was refluxed for 36 h, and then quenched by dropwise addition ofMeOH (70 mL). The mixture was evaporated in vacuo, and 10% aq NaOH (110 mL) and CH2Cl2(50 mL) were added to the residue. The resulting mixture was stirred for 30 min, then Boc2O (4.58 g,21 mmol) in CH2Cl2 (55 mL) was added, and the mixture was stirred virogously at rt for 1 d. Theorganic phase was separated, and the aqueous phase was extracted with CH2Cl2 (3100 mL). Thecombined organic phases were dried over MgSO4 and evaporated in vacuo. The residue wasrecrystallized from Hexanes to give 22 (2.13 g, 67%). White crystals. Mp 97-98 C. MS (m/z, CI): 228(MH+). IR (KBr, cm-1): 3449, 3448 ((O-H)), 1666 ((C=O)), 1408, 1176. Anal. calc. for C12H21NO3C 63.41, H 9.31, N 6.16. Found C 63.08, H 9.14, N 6.48. 1H NMR (CDCl3) 3.50 (br s, 2H), 3.47 (s,2H), 3.42 (s, 2H), 2.45 (br s, 0.5H), 2.38 (br s, 0.5H), 1.90 (br s, 2H), 1.80 (br s, 1H), 1.47 (s, 9H), 1.34(br s, 2H). 13C NMR (CDCl3) 156.5 (C), 79.4 (C), 67.8 and 67.4 (CH2), 52.2 and 51.8 (CH2), 50.2 and49.8 (CH2), 42.7 (C), 32.6 and 32.4 (CH2), 28.71 (CH3), 28.67 (CH).
References:
Tymtsunik, Andriy V.;Bilenko, Vitaliy A.;Grygorenko, Oleksandr O.;Komarov, Igor V. [Synlett,2014,vol. 25,# 3,art. no. ST-2013-B0898-L,p. 355 - 358] Location in patent:supporting information

24424-99-5
823 suppliers
$13.50/25G
![METHYL 2-OXO-3-AZABICYCLO[3.1.1]HEPTANE-1-CARBOXYLATE](/CAS/20180601/GIF/1628783-91-4.gif)
1628783-91-4
10 suppliers
inquiry
![tert-butyl 1-(hydroxymethyl)-3-azabicyclo[3.1.1]heptane-3-carboxylate](/CAS/20181022/GIF/1087784-78-8.gif)
1087784-78-8
19 suppliers
inquiry
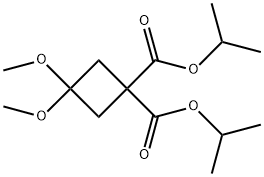
115118-68-8
146 suppliers
$13.00/1g
![tert-butyl 1-(hydroxymethyl)-3-azabicyclo[3.1.1]heptane-3-carboxylate](/CAS/20181022/GIF/1087784-78-8.gif)
1087784-78-8
19 suppliers
inquiry
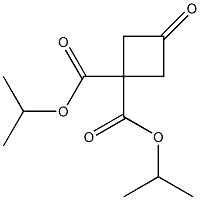
893724-10-2
56 suppliers
$10.00/1g
![tert-butyl 1-(hydroxymethyl)-3-azabicyclo[3.1.1]heptane-3-carboxylate](/CAS/20181022/GIF/1087784-78-8.gif)
1087784-78-8
19 suppliers
inquiry
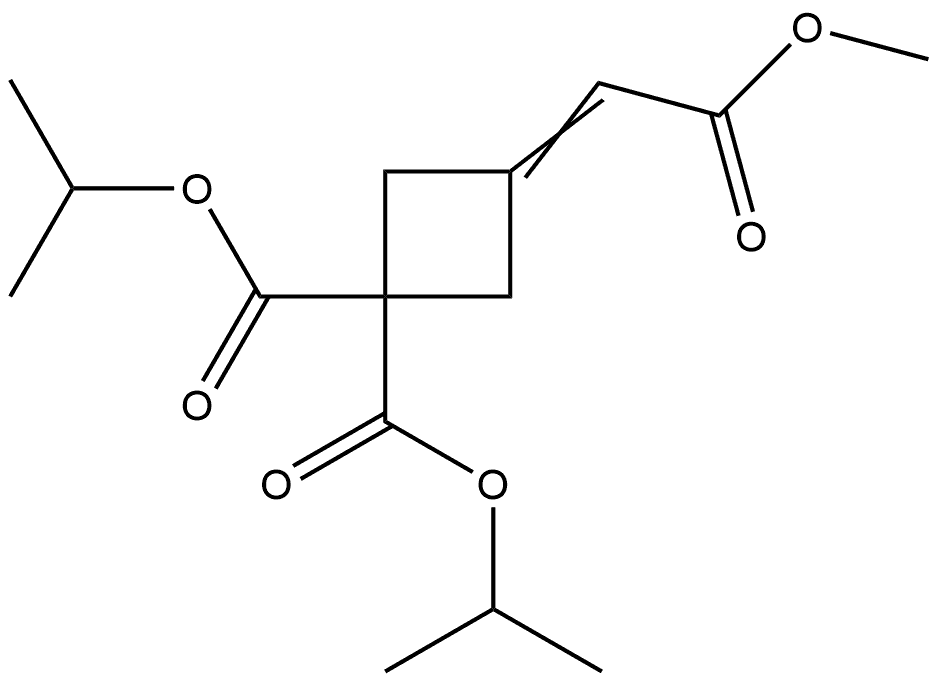
1628783-86-7
0 suppliers
inquiry
![tert-butyl 1-(hydroxymethyl)-3-azabicyclo[3.1.1]heptane-3-carboxylate](/CAS/20181022/GIF/1087784-78-8.gif)
1087784-78-8
19 suppliers
inquiry