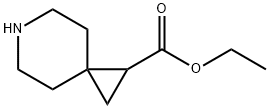
6-Azaspiro[2.5]octane-1-carboxylic acid ethyl ester synthesis
- Product Name:6-Azaspiro[2.5]octane-1-carboxylic acid ethyl ester
- CAS Number:1088498-24-1
- Molecular formula:C10H17NO2
- Molecular Weight:183.25
![6-(Benzyloxycarbonyl)-6-azaspiro[2.5]octane-1-carboxylic acid](/CAS2/GIF/147610-85-3.gif)
147610-85-3
37 suppliers
$70.00/250mg
![6-Azaspiro[2.5]octane-1-carboxylic acid ethyl ester](/CAS/20180629/GIF/1088498-24-1.gif)
1088498-24-1
9 suppliers
inquiry
Yield:1088498-24-1 44%
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in ethanol at 18 - 25;
Steps:
8.A
Example 8(4-Cyclobutylpiperazin-l-yl)(6-(tetrahydro-2H-pyran-4-yl)-6-azaspiro[2.5]octan-l- yl)methanone
References:
WO2008/147314,2008,A1 Location in patent:Page/Page column 55; 56
![6-benzyl 1-ethyl 6-azaspiro[2.5]octane-1,6-dicarboxylate](/CAS/20180702/GIF/147610-84-2.gif)
147610-84-2
28 suppliers
$240.00/500mg
![6-Azaspiro[2.5]octane-1-carboxylic acid ethyl ester](/CAS/20180629/GIF/1088498-24-1.gif)
1088498-24-1
9 suppliers
inquiry
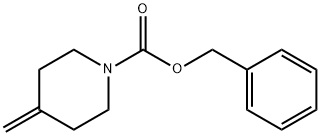
138163-12-9
103 suppliers
$18.00/1g
![6-Azaspiro[2.5]octane-1-carboxylic acid ethyl ester](/CAS/20180629/GIF/1088498-24-1.gif)
1088498-24-1
9 suppliers
inquiry