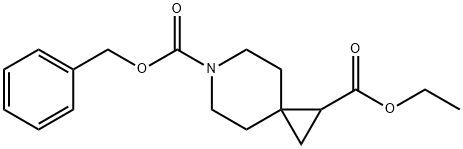
6-benzyl 1-ethyl 6-azaspiro[2.5]octane-1,6-dicarboxylate synthesis
- Product Name:6-benzyl 1-ethyl 6-azaspiro[2.5]octane-1,6-dicarboxylate
- CAS Number:147610-84-2
- Molecular formula:C18H23NO4
- Molecular Weight:317.38
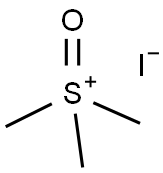
1774-47-6
537 suppliers
$6.00/25g

82244-11-9
17 suppliers
inquiry
![6-benzyl 1-ethyl 6-azaspiro[2.5]octane-1,6-dicarboxylate](/CAS/20180702/GIF/147610-84-2.gif)
147610-84-2
28 suppliers
$240.00/500mg
Yield:147610-84-2 75%
Reaction Conditions:
Stage #1: trimethylsulfoxonium iodidewith potassium tert-butylate in dimethyl sulfoxide at 20; for 2 h;
Stage #2: ethyl-1-carbobenzoxy-Δ4,α-piperidine-4-acetate in dimethyl sulfoxide at 20;
Steps:
1.B Step B
Potassium tert-butoxide (8.43 g, 75.1 mmol) was added to a solution of trimethylsulfoxonium iodide (17.5 g, 79.5 mmol) in DMSO (100 mL) in one portion. The mixture was stirred at RT for 2 h, after which benzyl 4-(2-ethoxy-2-oxoethylidene)piperidine- 1-carboxylate (13.4 g, 44.2 mmol) in DMSO (50 mL) was added. The mixture was stirred at RT overnight and then slowly added to a sat. aq. NH4C1 solution at 0 °C with stirring. The mixture was extracted with ether. The combined organic layers were washed with sat. aq. NaHCO3 solution and concentrated under reduced pressure. The residue was taken up in EtOAc (100 mL). Aqueous KMnO4 (2.0 gin 200 mL H20) and NaHCO3 (1.5 g) were added and the mixture was stirred overnight. The mixture was then filtered through Celite (J.T. Baker, Phillipsberg, NJ, diatomaceous earth) and extracted with EtOAc. The organic layer was washed with brine and dried over Na2SO4. The crude residue was purified by silica gel column chromatography (hexanes: EtOAc 5:1 to 4:1) to afford 6-benzyl 1-ethyl 6- azaspiro[2.5]octane-1,6-dicarboxylate (10.52 g, 75% yield). Mass Spectrum (ESI) m/z = 340 (M+23). ‘HNMR(400 IVIFlz, CDC13): ppm 0.93 (1H, dd, J=8.0, 4.6 Hz), 1.17 (1H, t, J5.0 Hz), 1.26 (3H, t, J=7.1 Hz), 1.40-1.46 (2H, m), 1.56 (1H, dd, J8.0, 5.4 Hz), 1.69-1.74 (2H, m), 3.31-3.39 (1H, m), 3.46- 3.61 (3H, m), 4.13 (2H, q, J=7.1 Hz), 5.13 (2H, s), 7.30-7.36 (5H, m).
References:
WO2017/83867,2017,A1 Location in patent:Paragraph 00180
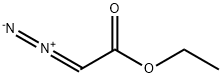
623-73-4
147 suppliers
$27.30/25ML
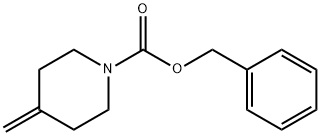
138163-12-9
108 suppliers
$18.00/1g
![6-benzyl 1-ethyl 6-azaspiro[2.5]octane-1,6-dicarboxylate](/CAS/20180702/GIF/147610-84-2.gif)
147610-84-2
28 suppliers
$240.00/500mg