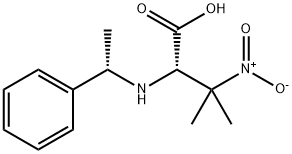
(S)-methyl 3-methyl-3-nitro-2-((S)-1-phenylethylamino)butanoate synthesis
- Product Name:(S)-methyl 3-methyl-3-nitro-2-((S)-1-phenylethylamino)butanoate
- CAS Number:1093192-04-1
- Molecular formula:C14H20N2O4
- Molecular Weight:280.32
Yield: 96%
Reaction Conditions:
Stage #1:(S)-3-methyl-3-nitro-2-(((S)-1-phenylethyl)amino)butanoic acid with caesium carbonate in N,N-dimethyl-formamide for 0.166667 h;Sonication for 1 min;
Stage #2:methyl iodide in N,N-dimethyl-formamide at 10 - 20; for 2.41667 h;
Steps:
Synthesis of 3-Methyl-3-nitro-2(S)-(1(S)-phenylethyl-amino)-butyric acid methyl ester (B-(S)). Into a dry 100 mL flask with a stirbar was placed the amino-acid A-(S)(9.77 g, 36.7 mmol) and cesium carbonate (12.55 g, 38.05 mmol, 1.05 eq.) under nitrogen. With rapid stirring the dimethylformarnide (37 mL) was added rapidly and stirred for 10 min, with sonication for 1 min. The reaction mixture was then cooled in a 10-15 °C water bath as the first portion of iodomethane (1.75 mL, two-thirds of the 2.63 mL) was added dropwise over 15 min. The bath was removed and the reaction was brought to room temperature over 20 min. The second portion of iodomethane (0.88 mL, one-third of the 2.63 mL) was added dropwise more slowly over 30 min. After the reaction was stirred for an additional 20 min, check of a sample by HPLC-MS showed a major product peak (5.89 min, MH+ = 281.7; 99.2% convn) with of a small amount of the SM (2.64 min, -0.75%). After the reaction was stirred for an additional 60 min, check of a sample by HPLC-MS showed complete conversion to the product peak. The reaction was then washed with EtOAc and water into a separatory funnel containing EtOAc (250 mL), water (70 mL) and 3 M aq HCl (12.8 mL, 38.4 mmol). The phases were shaken and the aqueous layer was adjusted to pH -7-8 and separated. The organic phase was washed with 3 % aq Li2SO4 (3 x 100 mL), half-saturated aq NaHCO3 (50 mL) and satd aq NaCl (2 x 100 mL), dried (Na2SO4), filtered and evaporated under reduced pressure with a heptane chaser to an amber oil. After placing under full vacuum for >4 h, the amino-ester B-(S) (9.88 g, 96 % yield) was obtained. Check of a sample by HPLC-MS showed a major product peak (3.32 min, MH+ = 281.7). LC-MS [M+H] 281.7 (C14H20N2O4+H, requires 281.33).
References:
ACHAOGEN, INC. WO2008/154642, 2008, A2 Location in patent:Page/Page column 244-245
2627-86-3
540 suppliers
$10.60/1gm:

1093192-04-1
5 suppliers
inquiry