
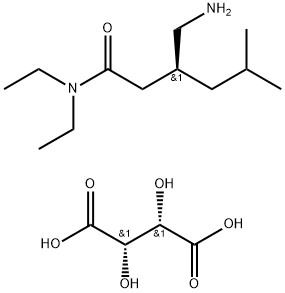
(3S)-3-(Aminomethyl)-N,N-diethyl-5-methylhexanamide synthesis
- Product Name:(3S)-3-(Aminomethyl)-N,N-diethyl-5-methylhexanamide
- CAS Number:1094517-97-1
- Molecular formula:C12H26N2O
- Molecular Weight:214.35
1094517-98-2
14 suppliers
inquiry

1094517-97-1
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium hydroxide in dichloromethane;water at 20 - 25; pH=9 - 10; for 0.166667 - 0.25 h;Product distribution / selectivity;
Steps:
7; 8
Example 7: Preparation of (S)-Pregabalin; The tartarate salt of 3- aminomethyl N,N-diethyl-5-methyl hexanamide(10 grams) was dissolved in water (15 ml) and dichloromethane (25 ml) was added to it.The pH of reaction mixture was adjusted to 9-10 by addition of sodium hydroxide solution (3.0 gm NaOH in 3.0 ml of water) and stirred for 10-15 min at 20-250C. The aqueous and organic layers were separated. The aqueous layer was extracted with
References:
WO2009/1372,2008,A2 Location in patent:Page/Page column 27-28