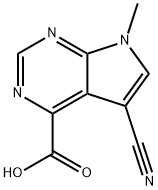
5-CYANO-7-METHYL-7H-PYRROLO[2,3-D]PYRIMIDINE-4-CARBOXYLIC ACID synthesis
- Product Name:5-CYANO-7-METHYL-7H-PYRROLO[2,3-D]PYRIMIDINE-4-CARBOXYLIC ACID
- CAS Number:1095822-79-9
- Molecular formula:C9H6N4O2
- Molecular Weight:202.17
Yield:1095822-79-9 83%
Reaction Conditions:
Stage #1: C9H6N4O2with sodium hydride in tetrahydrofuran at 0 - 20; for 1 h;
Stage #2: methyl iodide in tetrahydrofuran; for 16 h;
Stage #3: with hydrogenchloride;water
Steps:
17
Synthesis of Compound 17. Sodium hydride (60% in mineral oil, 350 mg, 8.9 mmol) was added to a solution of compound 16.5 (1.2 g, 5.9 mmol) in THF (12 ml) 0° C. The reaction mixture was wasmed to RT. After 1 hr, methyl iodide (1.1 ml, 17 mmol) was added to the reaction. After 16 hr, the reaction mixture was quenched with water (30 ml) and was then washed with ether (2×50 ml). The aqueous was acidified with 2N HCl, the precipitated material was filtered off to afford compound 17 (1 g, 83%) as an off white solid. 1H-NMR (500 MHz, DMSO-d6): δ 13.14 (bs, 1H), 9.08 (s, 1H), 8.75 (s, 1H), 3.90 (s, 3H). MS m/z 203 [M+1]+.
References:
US2009/5359,2009,A1 Location in patent:Page/Page column 25
![METHYL 5-CYANO-7H-PYRROLO[2,3-D]PYRIMIDINE-4-CARBOXYLATE](/CAS/20180601/GIF/1095822-75-5.gif)
