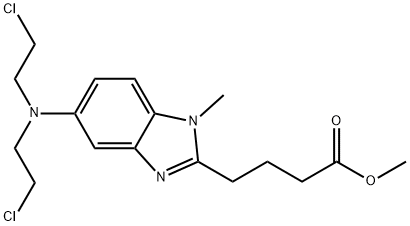
5-[Bis(2-chloroethyl)aMino]-1-Methyl-1H-benziMidazole-2-butanoic Acid Methyl Ester synthesis
- Product Name:5-[Bis(2-chloroethyl)aMino]-1-Methyl-1H-benziMidazole-2-butanoic Acid Methyl Ester
- CAS Number:109882-25-9
- Molecular formula:C17H23Cl2N3O2
- Molecular Weight:372.29
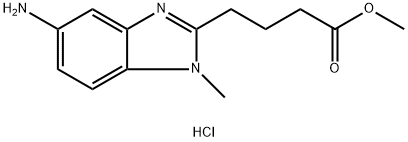
1221157-11-4
2 suppliers
inquiry
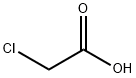
79-11-8
10 suppliers
$27.00/25g
![5-[Bis(2-chloroethyl)aMino]-1-Methyl-1H-benziMidazole-2-butanoic Acid Methyl Ester](/CAS/GIF/109882-25-9.gif)
109882-25-9
23 suppliers
$504.33/5MG
Yield:109882-25-9 100%
Reaction Conditions:
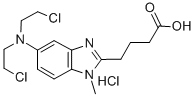
Stage #1: 4-(5-amino-1-methyl-1H-benzoimidazol-2-yl)butyric acid methyl ester hydrochloride;chloroacetic acid in tetrahydrofuran;Inert atmosphere;Heating;
Stage #2: with borane-THF in tetrahydrofuran at 20;
Stage #3: with methanol in tetrahydrofuran at 20;
Steps:
7
Example 7; Preparation of4-/5-fBis-(2-chloro-ethyl)-aminoJ-l-methyl-lH-benzoimidazol-2- ylj-butyric acid methyl ester. A one-liter, three neck, round bottom flask equipped with a stirring bar, condenser with nitrogen sweep, thermocouple with temperature controller, and heating mantle was charged with 4-(5 -amino- 1 -methyl- 1H-benzoimidazol-2-yl)- butyric acid methyl ester hydrochloride (15.0 g, 52.9 mmol. 1.0 eq), and chloroacetic acid (105.0 g, 21 eq.), and 22.5 mL of dry tetrahydrofuran (THF). The slurry was stirred in a tap water bath to allow all of the solids to be dissolved. Borane-THF (370.0 mL, 370 mmol, 7 eq.) was added slowly via an addition funnel. When the addition of BH3-THF was completed, the resulting reaction solution was stirred at room temperature for 5 hours and quenched with methanol at room temperature. The resulting solution was concentrated to approximately one-third weight by evaporation and neutralized to pH 7-8 with an aqueous sodium carbonate solution in an ice-water bath. A tan solid was collected by vacuum filtration then washed with water and methyl tert-butyl ether. After drying on the rotary evaporator with house vacuum at room temperature overnight, a tan solid was obtained in an essentially quantitative yield with a purity of 97.8 Area% (HPLC Method C). 1H NMR (400 MHz, DMSO-d6) δ 7.32 (d, J= 8.8 Hz, 1H), 6.92 (d, J= 2.3 Hz, 1H), 6.78 (dd, J= 8.8, 2.3 Hz, 1H), 3.70 (br s, 8H), 3.66 (s, 3H), 3.59 (s, 3H), 2.83 (t, J= 7.4 Hz, 2H), 2.48 (t, J= 7.4 Hz, 2H, overlapped partially with DMSO), 2.01 (quint, J= 7.4Hz, 2H); LC/MS (ESI, m/z) 372 (M+ 1), mp 60-63 °C dec.
References:
WO2010/42568,2010,A1 Location in patent:Page/Page column 19-20

67-56-1
736 suppliers
$9.00/25ml
3543-75-7
367 suppliers
$35.00/10mg
![5-[Bis(2-chloroethyl)aMino]-1-Methyl-1H-benziMidazole-2-butanoic Acid Methyl Ester](/CAS/GIF/109882-25-9.gif)
109882-25-9
23 suppliers
$504.33/5MG
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid methyl ester](/CAS/GIF/109882-31-7.gif)
109882-31-7
14 suppliers
inquiry
![5-[Bis(2-chloroethyl)aMino]-1-Methyl-1H-benziMidazole-2-butanoic Acid Methyl Ester](/CAS/GIF/109882-25-9.gif)
109882-25-9
23 suppliers
$504.33/5MG
![Pentanoic acid, 5-[(2,4-dinitrophenyl)amino]-5-oxo-, methyl ester](/CAS/20180808/GIF/1221157-09-0.gif)
1221157-09-0
1 suppliers
inquiry
![5-[Bis(2-chloroethyl)aMino]-1-Methyl-1H-benziMidazole-2-butanoic Acid Methyl Ester](/CAS/GIF/109882-25-9.gif)
109882-25-9
23 suppliers
$504.33/5MG
![Pentanoic acid, 5-[(2,4-dinitrophenyl)methylamino]-5-oxo-, methyl ester](/CAS/20200401/GIF/1221157-10-3.gif)
1221157-10-3
0 suppliers
inquiry
![5-[Bis(2-chloroethyl)aMino]-1-Methyl-1H-benziMidazole-2-butanoic Acid Methyl Ester](/CAS/GIF/109882-25-9.gif)
109882-25-9
23 suppliers
$504.33/5MG