
10H-Phenothiazine, 3,7-dibromo-10-methyl- synthesis
- Product Name:10H-Phenothiazine, 3,7-dibromo-10-methyl-
- CAS Number:34964-70-0
- Molecular formula:C13H9Br2NS
- Molecular Weight:371.09
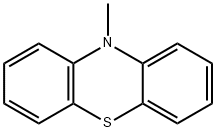
1207-72-3
109 suppliers
$20.00/1g

34964-70-0
20 suppliers
inquiry
Yield:34964-70-0 99%
Reaction Conditions:
with bromine;acetic acid at 20; for 13 h;Inert atmosphere;
Steps:
2.3.5. 3,7-di(bromo)-10H-MPT (6) (DBMPT)
MPT (10 g, 46.8 mmol) was dissolved in 75 mL glacial acetic aciddegassed beforehand for 30 minutes under argon. The mixture wasstirring under inert atmosphere at room temperature. Afirst molarequivalent of bromine (Br2) (2.43 mL, 46.8 mmol) was addeddropwise. After the system had been stirred for 1 h at roomtemperature, another portion of bromine (2.43 mL, 46.8 mmol)was added to the reaction mixture. The solution was stirred for 12 hat room temperature, and then a saturated aqueous solution ofsodium sulfite (40 mL) and diethyl ether (70 mL) were added to themixture and the system was stirred for 1 h. The organic phase wasseparated, and the aqueous layer was extracted three times withdiethyl ether. The combined organic layers were dried with sodiumsulfate, and the solvents were removed under reduced pressure.The residue was chromatographed on silica gel (ethyl acetate/hexane 2:3) to give the desired product which was recovered asgreen solid (17 g, 99% yield). 1H NMR ((CD3)2CO, 300 MHz) δ/ppm:3.37 (s, 3H), 6.90 (d, J = 8.6 Hz, 2H), 7.30 (d, J = 2.3 Hz, 2H), 7.36 (dd,J = 2.3 and 8.6 Hz, 2H). Elemental analysis: found (C, 42.41; H, 2.19;N, 3.43); calculated (C, 42.08; H, 2.44; N, 3.77).
References:
Guilmin, Romain;Alloin, Fannie;Molton, Florian;Leprêtre, Jean-Claude [Electrochimica Acta,2017,vol. 232,p. 182 - 191]
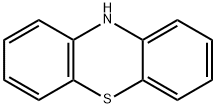
92-84-2
562 suppliers
$10.00/5g

34964-70-0
20 suppliers
inquiry

