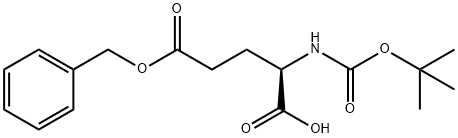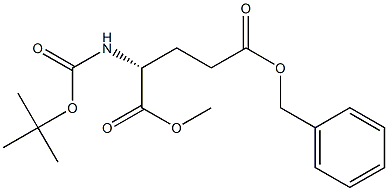
(R)-5-benzyl 1-methyl 2-(tert-butoxycarbonylamino)pentanedioate synthesis
- Product Name:(R)-5-benzyl 1-methyl 2-(tert-butoxycarbonylamino)pentanedioate
- CAS Number:110473-10-4
- Molecular formula:C18H25NO6
- Molecular Weight:351.3942
Yield:110473-10-4 98%
Reaction Conditions:
Stage #1: N-(tert-butyloxycarbonyl)-γ-benzyl-D-glutamic acidwith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.25 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 0; for 18 h;
Steps:
16 Intermediate 16 Boc-D-Glu(OBn)OMe
Intermediate 16 Boc-D-Glu(OBn)OMe Boc-D-TGlu(OBn)OH (5.0 g, 14.84 mmol) was dissolved in dry DMF (50 mL), then potassium carbonate (2.32 g, 16.81 mmol) was added, the mixture was stirred at rt for 15 min, then the mixture was cooled to 0°C and methyl iodide (4.21 g, 29.64 mmol) was added dropwise. The stirring was maintained for 18h. Then, the solvent was removed in vacuo and the residue was diluted with EtOAc (50 mL) and the organic layer was washed with water and brine dried over MgSCU, filtered and concentrated. The crude product was purified by flash column chromatography (silica gel, DCM/MeOH 0 to 1%) to give 5.12 g (14.58 mmol, 98% yield) of the title compound. *Η NMR (DMSO-c δ 7.38-7.31 (5H, m), 7.30 (IH, d), 5.09 (2H, s), 4.00 (IH, m), 3.62 (3H, s), 2.49 (2H, m), 1.99 (IH, m), 1.81 (IH, m), 1.38 (9H,s).
References:
WO2016/8946,2016,A1 Location in patent:Page/Page column 55-56
6893-26-1
456 suppliers
$6.00/10g

110473-10-4
9 suppliers
inquiry

100-51-6
1384 suppliers
$5.00/100g

110473-10-4
9 suppliers
inquiry