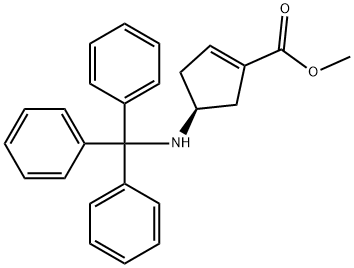
Methyl (4S)-4-(tritylamino)cyclopent-1-ene-1-carboxylate synthesis
- Product Name:Methyl (4S)-4-(tritylamino)cyclopent-1-ene-1-carboxylate
- CAS Number:1113025-21-0
- Molecular formula:C26H25NO2
- Molecular Weight:383.48
![2-Cyclopentene-1-carboxylic acid, 4-[(triphenylMethyl)aMino]-, Methyl ester, (1S,4R)-](/CAS/20150408/GIF/1113025-16-3.gif)
1113025-16-3
4 suppliers
inquiry

1113025-21-0
10 suppliers
inquiry
Yield:-
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in dichloromethane at 40; for 16 - 22 h;
Steps:
7 Methyl (4S)-4-(tritylamino)cyclopent-1-ene-1-carboxylate
A reactor was charged with a solution of methyl (1S,4R)-4-(tritylamino)cyclopent-2-ene-1-carboxylate (4.75 kg, 12.4 mol) in methylene chloride. The reactor was charged with additional methylene chloride (15 L) to bring the total volume to 23.8 L. To the stirred solution was added 1,8-diazabicyclo[5.4.0]undec-7-ene (4.82 L, 32.2 mol). The reaction mixture was warmed to 40° C., with stirring for 16 to 22 h. 1H NMR (CDCl3) analysis of a small sample of the reaction mixture confirmed the formation of the product. The reaction was washed with 10% aqueous citric acid solution (2×7 L). The organic phase was concentrated under reduced pressure to afford the title compound as an oil. The oil was diluted with anhydrous toluene and concentrated to remove residual water and used without further purification. 1H NMR (300 MHz, CDCl3, δ): 7.60-7.54 (m, 5H), 7.34-7.17 (m, 10H), 6.53-6.50 (m, 1H), 3.70 (s, 3H), 3.50-3.40 (m, 1H), 2.60-2.52 (dd, J=16.6, 8.3 Hz, 1H), 2.24-2.20 (m, 1H), 2.16-2.05 (m, 1H) and 1.91-1.80 (m, 1H).
References:
US2009/36678,2009,A1 Location in patent:Page/Page column 25
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
223 suppliers
$10.00/1g

1113025-21-0
10 suppliers
inquiry
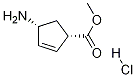
229613-83-6
42 suppliers
inquiry

1113025-21-0
10 suppliers
inquiry