
O-TERT-BUTYLDIMETHYLSILYL-N-METHYL-N-T-BUTOXYCARBONYL-L-TYROSINE, METHYL ESTER synthesis
- Product Name:O-TERT-BUTYLDIMETHYLSILYL-N-METHYL-N-T-BUTOXYCARBONYL-L-TYROSINE, METHYL ESTER
- CAS Number:112196-58-4
- Molecular formula:C22H37NO5Si
- Molecular Weight:423.62

112196-57-3
17 suppliers
$105.00/500mg

74-88-4
351 suppliers
$15.00/10g

112196-58-4
17 suppliers
$230.00/500mg
Yield:112196-58-4 436 mg
Reaction Conditions:
Stage #1: methyl (S)-2-((tert-butoxycarbonyl)amino)-3-(4-((tert-butyldimethylsilyl)-oxy)phenyl)propanoatewith sodium hydride in tetrahydrofuran;N,N-dimethyl-formamide; for 0.166667 h;
Stage #2: methyl iodide in tetrahydrofuran;N,N-dimethyl-formamide; for 24 h;
Steps:
131.C Step C.
Step C.
Methyl (S)-2-((tert-butoxycarbonyl)(methyl)amino)-3-(4-((tert-butyldimethylsilyl)oxy)phenyl)propanoate (Intermediate 131C)
Sodium hydride (72 mg, 1.80 mmol) was added to a solution of Intermediate 131B (692 mg, 1.69 mmol) in a mixture of THF (10 mL) and DMF (1 mL).
After stirring for 10 min, methyl iodide (316 mg, 5.08 mmol) was added and stirring was continued for 24 h.
The reaction mixture was partitioned between ethyl acetate (5 mL) and water (5 mL) and the organic layer was separated, washed with water (2*10 mL), dried (Na2SO4) and evaporated.
The product was purified on a Si cartridge (25 g) eluting with 0-25% ethyl acetate in cyclohexane to give the desired product as a yellow oil (436 mg). LCMS (Method 6): Rt=1.95 min, m/z 424.3 [M+H]+
References:
US2018/215758,2018,A1 Location in patent:Paragraph 0473

74-88-4
351 suppliers
$15.00/10g
![L-Tyrosine, N-[(1,1-dimethylethoxy)carbonyl]-O-[(1,1-dimethylethyl)dimethylsilyl]-N-methyl-](/CAS/20210305/GIF/848780-45-0.gif)
848780-45-0
0 suppliers
inquiry

112196-58-4
17 suppliers
$230.00/500mg
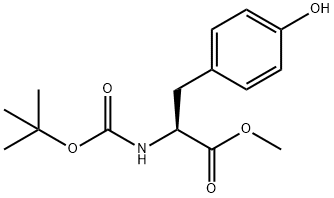
4326-36-7
368 suppliers
$6.00/5g

112196-58-4
17 suppliers
$230.00/500mg
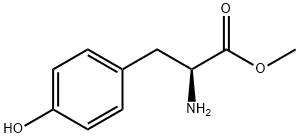
1080-06-4
288 suppliers
$6.00/5g

112196-58-4
17 suppliers
$230.00/500mg

3978-80-1
354 suppliers
$7.87/5g

112196-58-4
17 suppliers
$230.00/500mg