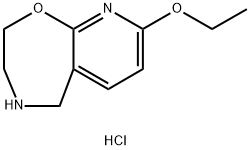
8-ethoxy-2,3,4,5-tetrahydropyrido[3,2-f][1,4]oxazepine hydrochloride synthesis
- Product Name:8-ethoxy-2,3,4,5-tetrahydropyrido[3,2-f][1,4]oxazepine hydrochloride
- CAS Number:1154473-06-9
- Molecular formula:C10H15ClN2O2
- Molecular Weight:230.69
![Pyrido[3,2-f]-1,4-oxazepine-4(5H)-carboxylic acid, 8-ethoxy-2,3-dihydro-, 1,1-diMethylethyl ester](/CAS/GIF/1154471-53-0.gif)
1154471-53-0
2 suppliers
inquiry
![8-ethoxy-2,3,4,5-tetrahydropyrido[3,2-f][1,4]oxazepine hydrochloride](/CAS/GIF/1154473-06-9.gif)
1154473-06-9
2 suppliers
inquiry
Yield:1154473-06-9 78%
Reaction Conditions:
with hydrogenchloride in ethyl acetate at 20; for 3 h;
Steps:
13.2
(step 2) A mixture of the compound obtained in step 1 (0.16 g) and 4N hydrogen chloride/ethyl acetate (3 mL) was stirred at room temperature for 3 hr. The precipitate was collected by filtration, and the aqueous layer was basified and extracted with ethyl acetate and aqueous sodium hydroxide solution. The organic layer was washed with saturated brine and dried, and the solvent was evaporated under reduced pressure. Ethyl acetate was added to the residue, and 4N hydrogen chloride/ethyl acetate was added. The precipitate was collected by filtration, and recrystallized from ethanol-diisopropyl ether to give the title compound (0.10 g, 78%) as a white powder. 1H-NMR(DMSO-d6):δ1.29(3H,t,J=7.0Hz), 3.40-3.47(2H,m), 4.20-4.24(4H,m), 4.31-4.34(2H,m), 6.59(1H,d,J=8.1Hz), 7.78(1H,d,J-8.1Hz), 9.60 (2H,brs) MS(ESI+):195(M-HCl+H)
References:
EP2213675,2010,A1 Location in patent:Page/Page column 37