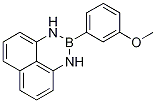
2-(3-Methyoxyphenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine synthesis
- Product Name:2-(3-Methyoxyphenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine
- CAS Number:1159803-56-1
- Molecular formula:C17H15BN2O
- Molecular Weight:274.12

1214264-88-6
59 suppliers
inquiry

536-90-3
289 suppliers
$10.00/5g
![2-(3-Methyoxyphenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine](/CAS/GIF/1159803-56-1.gif)
1159803-56-1
15 suppliers
$75.00/500mg
Yield:1159803-56-1 70%
Reaction Conditions:
with tert.-butylnitrite;sodium acetate;tetra-(n-butyl)ammonium iodide;dibenzoyl peroxide in acetonitrile at 80;Schlenk technique;Inert atmosphere;
Steps:
4.2. General Procedure for the Direct Transformation from Arylamines to Aryl Naphthalene-1,8-diamino Boronamides
General procedure: In air, Bpin-B(dan) (0.1 mmol, 1.0 eq.), aryl amide (0.2 mmol, 2.0 eq.), TBAI (0.01 eq.),NaOAc (0.15 eq.), and BPO (0.01 eq.) were sequentially weighed and added to a screw-cappedSchenk tube containing a magnetic stir bar. The vessel was evacuated and refilled with nitrogen forthree times. t-BuONO (0.2 eq.) and MeCN (0.6 mL) were added in turn under N2 atmosphere usingsyringes through a septum which was temporarily used to replace the screw cap. The reaction mixturewas then vigorously stirred at 80 °C for the indicated time. The resulting mixture was filtered through a pad of Celite, and the filter cake was washed with ethyl acetate (3 mL x 2). The combined filtratewas evaporated under vacuum to dryness and the residue was purified by column chromatography toyield the desired product.
References:
Ding, Siyi;Ma, Qiang;Zhu, Min;Ren, Huaping;Tian, Shaopeng;Zhao, Yuzhen;Miao, Zongcheng [Molecules,2019,vol. 24,# 3,art. no. 377]
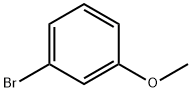
2398-37-0
576 suppliers
$8.00/10g

1214264-88-6
59 suppliers
inquiry
![2-(3-Methyoxyphenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine](/CAS/GIF/1159803-56-1.gif)
1159803-56-1
15 suppliers
$75.00/500mg

1214264-88-6
59 suppliers
inquiry
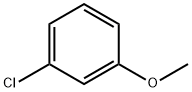
2845-89-8
283 suppliers
$6.00/5g
![2-(3-Methyoxyphenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine](/CAS/GIF/1159803-56-1.gif)
1159803-56-1
15 suppliers
$75.00/500mg
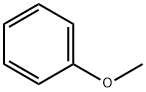
100-66-3
511 suppliers
$10.00/5G
![1H-Naphtho[1,8-de]-1,3,2-diazaborine, 2,3-dihydro-](/CAS/20211123/GIF/5656-71-3.gif)
5656-71-3
1 suppliers
inquiry
![2-(3-Methyoxyphenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine](/CAS/GIF/1159803-56-1.gif)
1159803-56-1
15 suppliers
$75.00/500mg
![2-(4-Methyoxyphenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine](/CAS/GIF/1159803-53-8.gif)
1159803-53-8
16 suppliers
$45.00/50mg