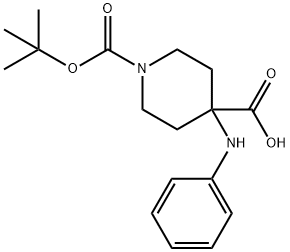
1-(tert-butoxycarbonyl)-4-(phenylaMino)piperidine-4-carboxylic acid synthesis
- Product Name:1-(tert-butoxycarbonyl)-4-(phenylaMino)piperidine-4-carboxylic acid
- CAS Number:1159835-31-0
- Molecular formula:C17H24N2O4
- Molecular Weight:320.38
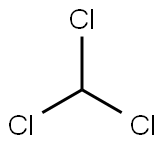
67-66-3
6 suppliers
$39.79/500ml

79099-07-3
543 suppliers
$5.00/5g

62-53-3
638 suppliers
$10.00/1g

1159835-31-0
22 suppliers
inquiry
Yield:1159835-31-0 78.93%
Reaction Conditions:
with sodium hydroxide at 0 - 20; for 19 h;
Steps:
1-(Tert-butoxycarbonyl)-4-(phenylamino)piperidine-4-carboxylic acid (01)
To a solution of aniline (50 mL, 0.567 mol) in THF (4.0 L), NaOH (111.1 g, 2.778 mol), N-Boc-4-piperidone (331.5 g, 1.667 mol) and CH3Cl (214.8 mL, 2.778 mol, 5equiv) were added in an ice bath at 0 °C, and the reaction mixture was stirred at 0 °C for 1h and then at room temperature for 18 h. The reaction mixture was filtered through a Buchner funnel, and the filter cake was washed with THF. The filter cake was dissolved in water, and the solution was washed with ethyl acetate. The aqueous layer was acidified to pH 2-3 with a 1N HCl solution. The mixture was extracted with ethyl acetate (1000 mL × 3), and the organic phases were washed with saturated NaCl solution (1000 mL × 1). The organic layer was dried over Na2SO4, filtered, and evaporated to afford 143.76 g (78.93%) of compound 01.
References:
Li, Jing;Zhang, Tao;Sun, Jialin;Ren, Fengxia;Jia, Hongxin;Yu, Zixing;Cheng, Jingchao;Shi, Weiguo [Chinese Chemical Letters,2022,vol. 33,# 8,p. 4107 - 4110] Location in patent:supporting information

79099-07-3
543 suppliers
$5.00/5g
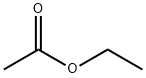
141-78-6
609 suppliers
$24.69/250ml

62-53-3
638 suppliers
$10.00/1g

1159835-31-0
22 suppliers
inquiry