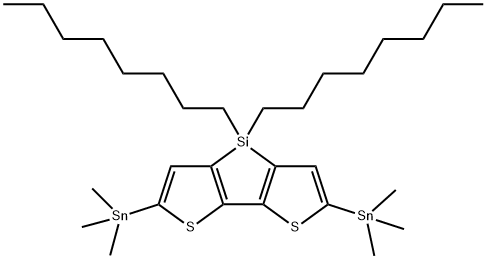
2,6-Di3MeTin-4,4-dioctyl-4H-silolo[3,2-b:4,5-b']dithiophene synthesis
- Product Name:2,6-Di3MeTin-4,4-dioctyl-4H-silolo[3,2-b:4,5-b']dithiophene
- CAS Number:1160106-15-9
- Molecular formula:C30H54S2SiSn2
- Molecular Weight:744.39
![4,4-Dioctyl-4H-silolo[3,2-b:4,5-b']dithiophene](/CAS/GIF/1160106-12-6.gif)
1160106-12-6
82 suppliers
$67.00/100mg

1066-45-1
147 suppliers
$31.50/2g
![2,6-Di3MeTin-4,4-dioctyl-4H-silolo[3,2-b:4,5-b']dithiophene](/CAS/20200611/GIF/1160106-15-9.gif)
1160106-15-9
22 suppliers
inquiry
Yield:1160106-15-9 95%
Reaction Conditions:
Stage #1: 4,4′-bis-(n-octyl)-dithieno[3,2-b:2′,3′-d]silolwith n-butyllithium in tetrahydrofuran at -78; for 1 h;Inert atmosphere;
Stage #2: trimethyltin(IV)chloride in tetrahydrofuran at -78 - 20;Inert atmosphere;
Steps:
1.3 3) Synthesis of Compound 3:
Take a double-neck round bottom flask, anhydrous and anaerobic treatment, anhydrous tetrahydrofuran as solvent, nitrogen protectionAdd dithieno-silacyclopentane under the protection, add n-butyllithium at -78 ° C, stir at -78 ° C for one hour, then add trimethyltin chloride, return to room temperature and stir, reaction overnight, TLC monitoring After the reaction is completed, the reaction solution is quenched by adding a potassium fluoride solution, and the reaction solution is transferred to a separating funnel, and the organic phase is washed with dichloromethane and brine, and dried over anhydrous sodium sulfate. After concentration by pressure, the compound 3 is obtained as a yellow oil. The ratio of the dithienosilane, n-butyllithium, trimethyltin chloride and tetrahydrofuran is: dithienosilyl 1 weight Share:2.2 parts by weight of n-butyllithium: 2.4 parts by weight of trimethyltin chloride: 40 parts by volume of tetrahydrofuran, yield 95%;
References:
CN109293693,2019,A Location in patent:Paragraph 0059; 0064; 0067
492-97-7
316 suppliers
$5.00/1g
![2,6-Di3MeTin-4,4-dioctyl-4H-silolo[3,2-b:4,5-b']dithiophene](/CAS/20200611/GIF/1160106-15-9.gif)
1160106-15-9
22 suppliers
inquiry
125143-53-5
129 suppliers
$5.00/250mg
![2,6-Di3MeTin-4,4-dioctyl-4H-silolo[3,2-b:4,5-b']dithiophene](/CAS/20200611/GIF/1160106-15-9.gif)
1160106-15-9
22 suppliers
inquiry
207742-50-5
95 suppliers
$42.00/100mg
![2,6-Di3MeTin-4,4-dioctyl-4H-silolo[3,2-b:4,5-b']dithiophene](/CAS/20200611/GIF/1160106-15-9.gif)
1160106-15-9
22 suppliers
inquiry
![4H-Silolo[3,2-b:4,5-b']dithiophene, 4,4-dioctyl-2,6-bis(trimethylsilyl)-](/CAS/20200611/GIF/1203451-18-6.gif)
1203451-18-6
0 suppliers
inquiry
![2,6-Di3MeTin-4,4-dioctyl-4H-silolo[3,2-b:4,5-b']dithiophene](/CAS/20200611/GIF/1160106-15-9.gif)
1160106-15-9
22 suppliers
inquiry