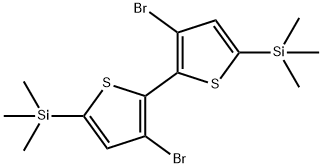
3,3'-Dibromo-5,5'-bis(trimethylsilyl)-2,2'-bithiophene synthesis
- Product Name:3,3'-Dibromo-5,5'-bis(trimethylsilyl)-2,2'-bithiophene
- CAS Number:207742-50-5
- Molecular formula:C14H20Br2S2Si2
- Molecular Weight:468.42
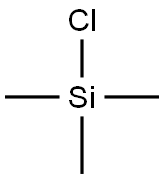
75-77-4
566 suppliers
$5.04/10
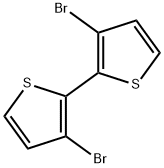
51751-44-1
233 suppliers
$7.00/100mg

207742-50-5
95 suppliers
$42.00/100mg
Yield: 92%
Reaction Conditions:
Stage #1:3,3'dibromo-2,2'-bithiophene with lithium diisopropyl amide in tetrahydrofuran;n-heptane at -78 - -20; for 0.25 h;Inert atmosphere;
Stage #2:chloro-trimethyl-silane in tetrahydrofuran;n-heptane at -78 - 0; for 1 h;
Steps:
4 Example 4
3,3'-dibromo-5,5'-di-trimethylsilyl-2,2'-dithiophene
Example 4
3,3'-dibromo-5,5'-di-trimethylsilyl-2,2'-dithiophene
To a freshly prepared LDA solution (82 ml butyllithium [2.7 m in heptane], 22.6 g di-isopropyl amine and 300 ml dry THF) at -78° C. under a nitrogen atmosphere, a solution of 32.4 g 3,3'-dibromo-2,2'-dithiophene 13 in 150 ml of dry THF is slowly added.
The solution is slowly warmed to -20° C., stirred for 15 minutes and then re-cooled to -78° C. 27.2 g trimethyl silylchloride is added at once and the solution is slowly allowed to warm to 0° C.
After stirring for 1 hour at 0° C. the reaction mixture is quenched by adding 100 ml water.
The phases are separated and the organic phase is washed twice with brine and dried over sodium sulphate.
The residue is suspended in methanol and the formed solid is recovered by filtration and dried under vacuum.
Affords 43 g (92%) of the title compound 15 as an off-white powder.
References:
BASF SE;Kirner, Hans Jürg;Bienewald, Frank;Flores, Jean-Charles;Aebischer, Olivier Frédéric US8436208, 2013, B2 Location in patent:Page/Page column 38
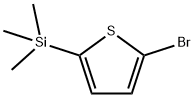
18246-28-1
36 suppliers
$202.00/1g

207742-50-5
95 suppliers
$42.00/100mg

75-77-4
566 suppliers
$5.04/10
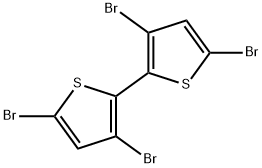
125143-53-5
129 suppliers
$5.00/250mg

207742-50-5
95 suppliers
$42.00/100mg

51751-44-1
233 suppliers
$7.00/100mg

1066-45-1
143 suppliers
$24.10/2g

207742-50-5
95 suppliers
$42.00/100mg
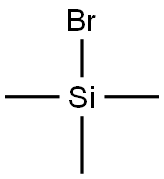
2857-97-8
417 suppliers
$7.00/25g

125143-53-5
129 suppliers
$5.00/250mg

207742-50-5
95 suppliers
$42.00/100mg