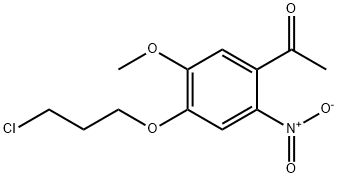
1-(4-(3-Chloropropoxy)-5-methoxy-2-nitrophenyl)ethanone synthesis
- Product Name:1-(4-(3-Chloropropoxy)-5-methoxy-2-nitrophenyl)ethanone
- CAS Number:1160707-44-7
- Molecular formula:C12H14ClNO5
- Molecular Weight:287.7
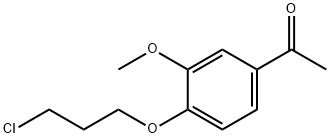
58113-30-7
123 suppliers
$55.00/10mg

1160707-44-7
6 suppliers
inquiry
Yield:1160707-44-7 89%
Reaction Conditions:
with HNO3 in dichloromethane at -20 - -10; for 2 h;
Steps:
1.B Example 1 N-[3-fluoro-4-[6-methoxy-7-[3-(tetrahydropyrrol-1-yl)propyloxy]quinolin-4-oxy]phenyl]-2-phenyl-4-methyl-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carboxamide Step B 1-[4-(3-chloropropyloxy)-5-methoxy-2-nitro]phenylethanone (III)
1-[4-(3-chloropropyloxy)-5-methoxy-2-nitro]phenylethanone (III)
Intermediate II (200 g, 0.82 Mol) was added to CH2Cl2 (5v/w, 1000 mL).
The mixture was stirred thoroughly so as to dissolve Intermediate II completely.
Then, after cooling the reaction solution to -20° C., fuming nitric acid (130 g, 2.06 mol) was slowly added dropwise thereto while keeping the temperature of the reaction solution below -10° C.
After the dropwise addition, the reaction was conducted at -20° C. for 2 h.
After completion of the reaction, the reaction solution was poured into ice-water.
The organic layer was collected, and washed with a saturated saline solution until the aqueous layer became neutral.
The organic layer was collected and dried over anhydrous sodium sulfate.
Then the solvent was evaporated off to produce a reddish brown oil, which was thoroughly cooled to produce a yellow solid (210 g) in a yield of 89%.
References:
US2013/252958,2013,A1 Location in patent:Paragraph 0087

58113-30-7
123 suppliers
$55.00/10mg
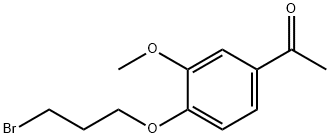
3245-49-6
35 suppliers
inquiry
![Ethanone, 1-[4-(3-bromopropoxy)-5-methoxy-2-nitrophenyl]-](/CAS/20180808/GIF/1184831-17-1.gif)
1184831-17-1
0 suppliers
inquiry

1160707-44-7
6 suppliers
inquiry

498-02-2
467 suppliers
$5.00/1g

1160707-44-7
6 suppliers
inquiry