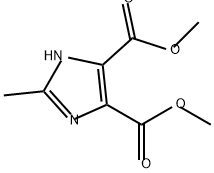
Dimethyl 1,2-dimethyl-1H-imidazole-4,5-dicarboxylate synthesis
- Product Name:Dimethyl 1,2-dimethyl-1H-imidazole-4,5-dicarboxylate
- CAS Number:117120-98-6
- Molecular formula:C9H12N2O4
- Molecular Weight:212.2
Yield:117120-98-6 88.3%
Reaction Conditions:
Stage #1: 2-Methylimidazol-4,5-dicarbonsaeure-dimethylesterwith potassium tert-butylate in N,N-dimethyl-formamide at 20; for 0.25 h;Inert atmosphere;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 20; for 2 h;Inert atmosphere;
Steps:
8.1 Step 1:
1,2-Dimethyl-1H-imidazole-4,5-dicarboxylic acid dimethyl ester
General procedure: To a suspension of dimethyl 2-methyl-1H-imidazole-4,5-dicarboxylate (2 g, 10.1 mmol, prepared according to J. Med. Chem. 1989, 32, 119) in DMF (20 mL) was added under a nitrogen atmosphere potassium tert-butoxide (1.25 g, 11.1 mmol) at room temperature.
The mixture was stirred for 15 min and iodomethane (694 μL, 11.1 mmol) was added.
The reaction mixture was stirred for 2 h at the same temperature.
The reaction mixture was poured into water (75 mL) and extracted with ethyl acetate (2*100 mL).
The organic phase was washed water (3*50 mL) and brine (20 mL), dried and concentrated in vacuo to give 720 mg of crude product.
The combined aqueous phases were back-extracted dichloromethane (4*50 mL), dried over MgSO4 and concentrated in vacuo to give another 1.4 g of crude product.
The combined crude material was purified by flash chromatography (using silica gel and a MeOH/dichloromethane gradient) to give the product as colorless oil (1.89 g, 8.91 mmol, 88.3%). MS: M=213.1 (M+H)+
References:
US2013/59833,2013,A1 Location in patent:Paragraph 0199
5313-35-9
33 suppliers
$109.00/50mg

117120-98-6
6 suppliers
inquiry