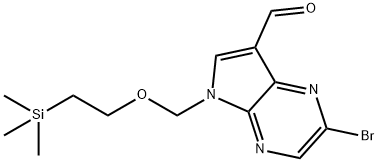
2-bromo-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine-7-carbaldehyde synthesis
- Product Name:2-bromo-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine-7-carbaldehyde
- CAS Number:1185428-34-5
- Molecular formula:C13H18BrN3O2Si
- Molecular Weight:356.29
![2-BroMo-5H-pyrrolo[2,3-b]pyrazine-7-carboxaldehyde](/CAS/20150408/GIF/1185428-32-3.gif)
1185428-32-3
55 suppliers
$137.00/100mg
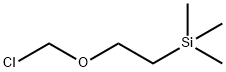
76513-69-4
354 suppliers
$6.00/1g
![2-bromo-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine-7-carbaldehyde](/CAS/20180703/GIF/1185428-34-5.gif)
1185428-34-5
30 suppliers
inquiry
Yield:1185428-34-5 53%
Reaction Conditions:
Stage #1: 2-bromo-5H-pyrrolo[2,3-b]pyrazine-7-carbaldehydewith sodium hydride in N,N-dimethyl-d6-formamide;mineral oil at 0 - 20; for 0.5 h;
Stage #2: (2-trimethylethylsilylethoxy)methyl chloride in N,N-dimethyl-formamide;mineral oil at 0 - 20; for 1 h;
Steps:
1.A.3 2-Bromo-5-(2-trimethylsilanyl-ethoxymethyl)-5H-pyrrolor2,3-blpyrazine-7-carbaldehyde
A stock solution of Jones reagent (2.67 M) was prepared by carefully adding concentrated H2S04 (2.3 mL) to Cr03 (2.67 g) then diluting to 10 mL with H20. To a partial suspension of (2- bromo-5H-pyrrolo[2,3-b]pyrazin-7-yl)-methanol (4.6 g, 20.1 mmol) in acetone (300 mL) was slowly added Jones reagent (9 mL, 24.0 mmol). During the addition the starting material gradually dissolved and a thick green precipitate was formed. The reaction mixture was stirred for 15 min then quenched with i-PrOH (2 mL) and filtered over Celite, rinsing with acetone. The filtrate was concentrated to provide 4.76 g of 2-bromo-5H-pyrrolo[2,3-b]pyrazine-7- carbaldehyde as a yellow-orange solid that was used without further purification. To a solution of this solid in DMF (50 mL) at 0°C was added NaH (60% in mineral oil, 1.2 g, 30.1 mmol). The reaction mixture was stirred at room temperature for 30 min then cooled back to 0°C and 2- (trimethylsilyl)ethoxymethyl chloride (4.3 mL, 24.1 mmol) was slowly added. The reaction mixture was warmed to room temperature and stirred for 1 h then quenched with H20 and extracted with EtOAc (3x). The combined organics were washed with H20 (3x) and brine then dried over MgS04 and concentrated. The residue was purified by Si02 chromatography (20% to 30% EtOAc/hexanes) to isolate 3.82 g (53%) of 2-bromo-5-(2-trimethylsilanyl-ethoxymethyl)- 5H-pyrrolo[2,3-b]pyrazine-7-carbaldehyde as a yellow solid. 1H NMR (CDC13, 300 MHz): δ (ppm) 10.37 (s, 1H), 8.50 (s, 1H), 8.33 (s, 1H), 5.73 (s, 2H), 3.53 - 3.70 (m, 2H), 0.90 - 1.05 (m, 2H), 0.00 (s, 9H).
References:
WO2014/29732,2014,A1 Location in patent:Page/Page column 38; 39
![5H-Pyrrolo[2,3-b]pyrazine, 2-bromo-5-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20210305/GIF/1184926-29-1.gif)
1184926-29-1
0 suppliers
inquiry

149409-22-3
3 suppliers
inquiry
![2-bromo-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine-7-carbaldehyde](/CAS/20180703/GIF/1185428-34-5.gif)
1185428-34-5
30 suppliers
inquiry
![2-Chloro-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine-7-carbaldehyde](/CAS/20180703/GIF/1334674-89-3.gif)
1334674-89-3
3 suppliers
inquiry

875781-43-4
254 suppliers
$19.00/1g
![2-bromo-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine-7-carbaldehyde](/CAS/20180703/GIF/1185428-34-5.gif)
1185428-34-5
30 suppliers
inquiry
![(2-BROMO-5H-PYRROLO[2,3-B]PYRAZINE-5,7-DIYL)DIMETHANOL](/CAS/20180529/GIF/1334674-87-1.gif)
1334674-87-1
5 suppliers
inquiry
![2-bromo-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine-7-carbaldehyde](/CAS/20180703/GIF/1185428-34-5.gif)
1185428-34-5
30 suppliers
inquiry
![(2-bromo-5H-pyrrolo[2,3-b]pyrazin-7-yl)methanol](/CAS/GIF/1334674-88-2.gif)
1334674-88-2
16 suppliers
$260.00/250mg
![2-bromo-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine-7-carbaldehyde](/CAS/20180703/GIF/1185428-34-5.gif)
1185428-34-5
30 suppliers
inquiry