
[5-(2,2,2-TRIFLUORO-1,1-DIMETHYL-ETHYL)-ISOXAZOL-3-YL]-CARBAMIC ACID PHENYL ESTER synthesis
- Product Name:[5-(2,2,2-TRIFLUORO-1,1-DIMETHYL-ETHYL)-ISOXAZOL-3-YL]-CARBAMIC ACID PHENYL ESTER
- CAS Number:1188911-77-4
- Molecular formula:C14H13F3N2O3
- Molecular Weight:314.26

1188911-74-1
33 suppliers
$1680.00/1g
1885-14-9
335 suppliers
$10.00/1g
![[5-(2,2,2-TRIFLUORO-1,1-DIMETHYL-ETHYL)-ISOXAZOL-3-YL]-CARBAMIC ACID PHENYL ESTER](/CAS/GIF/1188911-77-4.gif)
1188911-77-4
10 suppliers
inquiry
Yield:-
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 30; for 1 h;
Steps:
3.1 Procedure for synthesis of phenyl N-[5-(2,2,2-trifluoro-1,1-dimethyl-ethyl)isoxazol-3-yl]carbamate (Step 1)
Procedure for synthesis of phenyl N-[5-(2,2,2-trifluoro-1,1-dimethyl-ethyl)isoxazol-3-yl]carbamate (Step 1)
5-(2,2,2-trifluoro-1,1-dimethyl-ethyl)isoxazol-3-amine (for a synthesis see Journal of Medicinal Chemistry, 55(3), 1082-1105; 2012) (1.8 g, 9.3 mmol) in DCM (900 mL) was treated with N,N'-diisopropylethylamine (1.8 mL, 10 mmol) and the mixture stirred until homogeneous.
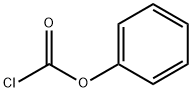
Phenyl chloroformate (1.6 g, 10 mmol) was charged to a dropping funnel and added to the reaction dropwise over 1 h, keeping temperature below 30° C.
This reaction mixture was used as is for reaction with amines-such as in examples A1-A4.
References:
Syngenta Participations AG;MORRIS, James Alan;PHADATE, Mangala;SONAWANE, Ravindra;RUSSELL, Claire Janet;MOSELEY, Donn Warwick;LONGSTAFF, Adrian;HOTSON, Matthew Brian;HENNESSY, Alan Joseph US2017/318810, 2017, A1 Location in patent:Paragraph 0226; 0227; 0228