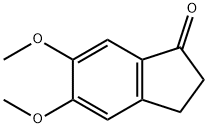
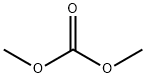
Methyl 5,6-dimethoxy-1-oxo-2,3-dihydro-1H-indene-2-carboxylate synthesis
- Product Name:Methyl 5,6-dimethoxy-1-oxo-2,3-dihydro-1H-indene-2-carboxylate
- CAS Number:119035-03-9
- Molecular formula:C13H14O5
- Molecular Weight:250.25
Yield:119035-03-9 100%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;mineral oil;Reflux;Inert atmosphere;
Steps:
3. General procedure for the acylation reactions
General procedure: In a round-bottomed flask NaH 60% in grease (3 eq.) was placed. Then, it wasdissolved in dry THF (0.2 mL/mmol NaH) while stirring under argon atmosphere,forming a white suspension. Next, dimethyl carbonate (3 eq.) was added (methyl 1Himidazole-1-carboxylate in case 1i) to the suspension and the corresponding ketone (1eq.) was dissolved with dry THF (1 mL/mol ketone) in an addition funnel. The ketonesolution was added to the reaction mixture dropwise in a period of 3-5 minutes. Thereaction mixture was heated to reflux until total conversion of the reagent was observedby TLC (3-5 h). Afterwards, 1 M HCl was added to the mixture until pH = 2-3. Theaqueous mixture was then extracted with dichloromethane. The organic fraction wasdried over anhydrous Na2SO4 and the solvent was removed under reduced pressure. Finally, the product was purified by column chromatography on silica-gel whennecessary.
References:
Granados, Albert;Sarró, Pau;Vallribera, Adelina [Molecules,2019,vol. 24,# 6,art. no. 1141] Location in patent:supporting information
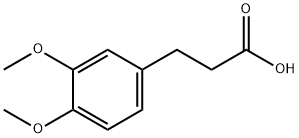
2107-70-2
290 suppliers
$6.00/5g

119035-03-9
11 suppliers
inquiry
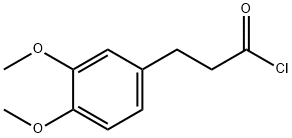
51842-87-6
10 suppliers
$220.00/500mg

119035-03-9
11 suppliers
inquiry