
1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridin-4-ylboronic acid synthesis
- Product Name:1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridin-4-ylboronic acid
- CAS Number:1190699-11-6
- Molecular formula:C16H27BN2O2Si
- Molecular Weight:318.29
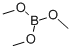
121-43-7
345 suppliers
$14.00/25mL
![1H-Pyrrolo[2,3-b]pyridine, 4-bromo-1-[tris(1-methylethyl)silyl]-](/CAS/GIF/640735-24-6.gif)
640735-24-6
30 suppliers
$104.00/500mg
![1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridin-4-ylboronic acid](/CAS/GIF/1190699-11-6.gif)
1190699-11-6
5 suppliers
inquiry
Yield:1190699-11-6 56%
Reaction Conditions:
Stage #1: Trimethyl borate;4-bromo-1-[tris(propan-2-yl)silyl]-1H-pyrrolo[2,3-b]pyridinewith n-butyllithium in tetrahydrofuran at -78; for 0.0833333 h;Inert atmosphere;
Stage #2: with n-butyllithium in tetrahydrofuran;hexane at -78; for 1 h;
Steps:
3.II Part II- Synthesis of (1-(Triisopropylsilyl)- 1H-pyrrolo[2,3-b]pyridin-4-yl)boronic acid
Into a 50 mL, 3 -necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen, was placed 4-bromo-1-(triisopropylsilyl)-1H-pyrrolo[[2,3-b]pyridine (400 mg, 1.13 mmol), tetrahydrofuran (20 mL) and trimethyl borate (233 mg, 2.24 mmol). The resulting solution was stirred for 5 min at -78 °C, then a solution of n-butyl lithium (1.4 mL, 3.5 mmol) in hexane was added into the flask. The resulting solution was allowed to react, with stirring, for an additional 1 hour while the temperature was maintained at -78 °C. The reaction was then quenched by the addition of 50 mL of NLLCl (aq). The resulting mixture was extracted with ethyl acetate, and the organic layers were combined and concentrated under vacuum. The residue was purified by column chromatography eluting with ethyl acetate/petroleum ether (1 :2). The collected fractions were combined and concentrated under vacuum to yield (1-(triisopropylsilyl)-1H-pyrrolo[2.3-b]pyridin-4-yl)boronic acid (200 mg, 56%).
References:
WO2019/200120,2019,A1 Location in patent:Paragraph 000408
![1H-Pyrrolo[2,3-b]pyridine, 4-bromo-1-[tris(1-methylethyl)silyl]-](/CAS/GIF/640735-24-6.gif)
640735-24-6
30 suppliers
$104.00/500mg
![1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridin-4-ylboronic acid](/CAS/GIF/1190699-11-6.gif)
1190699-11-6
5 suppliers
inquiry
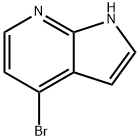
348640-06-2
325 suppliers
$6.00/250mg
![1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridin-4-ylboronic acid](/CAS/GIF/1190699-11-6.gif)
1190699-11-6
5 suppliers
inquiry
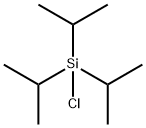
13154-24-0
414 suppliers
$20.00/10g
![1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridin-4-ylboronic acid](/CAS/GIF/1190699-11-6.gif)
1190699-11-6
5 suppliers
inquiry