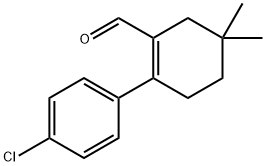
1-Cyclohexene-1-carboxaldehyde, 2-(4-chlorophenyl)-5,5-diMethyl- synthesis
- Product Name:1-Cyclohexene-1-carboxaldehyde, 2-(4-chlorophenyl)-5,5-diMethyl-
- CAS Number:1202186-71-7
- Molecular formula:C15H17ClO
- Molecular Weight:248.75
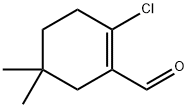
1053265-66-9
19 suppliers
inquiry
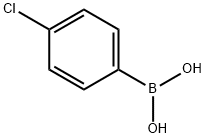
1679-18-1
595 suppliers
$10.00/1g

1202186-71-7
12 suppliers
inquiry
Yield:1202186-71-7 77%
Reaction Conditions:
with potassium carbonate;tetrakis(triphenylphosphine) palladium(0) in 1,4-dioxane;water at 100; for 0.2 h;Inert atmosphere;Microwave irradiation;
Steps:
A
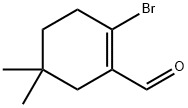
A suspension of 2-bromo-5,5-dimethylcyclohex- 1 -enecarbaldehyde(250 mg, 1.15 mmol), 4-chlorophenyl boronic acid (270 mg, 1.727 mmol) and Pd(Ph3P)4 (66.5 mg, 0.058 mmol) in dioxane (2.5 mL) was stirred at room temperature in a 5 mL microwave vial under an atmosphere of nitrogen. A solution of potassium carbonate (318 mg, 2.3 mmol) in water (0.3 mL) was added and the solution became clear. The vial was capped and heated to 100 °C for 12 minutes in a microwave. The reaction was cooled to room temperature, diluted with CH2CI2, filtered through a plug of Celite, andconcentrated. The crude residue was purified by flash chromatography on silica gel (0- 25% EtOAc/ heptanes) to afford 2-(4-chlorophenyl)-5,5-dimethyIcyclohex-l - enecarbaldehyde as a clear colorless oil (220mg, 77% yield).
References:
WO2011/29842,2011,A1 Location in patent:Page/Page column 59-60
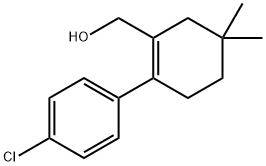
1027345-21-6
12 suppliers
$120.00/25mg

1202186-71-7
12 suppliers
inquiry
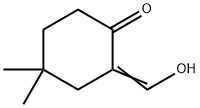
64230-02-0
0 suppliers
inquiry
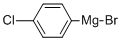
873-77-8
144 suppliers
$61.21/100ml

1202186-71-7
12 suppliers
inquiry
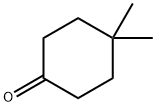
4255-62-3
201 suppliers
$10.00/1g

1202186-71-7
12 suppliers
inquiry