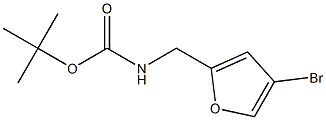
Carbamic acid,N-[(4-bromo-2-furanyl)methyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid,N-[(4-bromo-2-furanyl)methyl]-, 1,1-dimethylethyl ester
- CAS Number:1204655-02-6
- Molecular formula:C10H14BrNO3
- Molecular Weight:276.1271
21921-76-6
155 suppliers
$15.00/250mg

24424-99-5
871 suppliers
$13.50/25G
![Carbamic acid,N-[(4-bromo-2-furanyl)methyl]-, 1,1-dimethylethyl ester](/CAS/20180703/GIF/1204655-02-6.gif)
1204655-02-6
3 suppliers
inquiry
Yield:1204655-02-6 79%
Reaction Conditions:
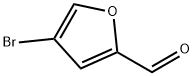
Stage #1: 4-bromo-2-furancarbaldehydewith hydroxylamine hydrochloride;sodium acetate in ethanol;water at 100; for 1 h;
Stage #2: with acetic acid;zinc at 20; for 1.91667 h;Cooling with ice;
Stage #3: di-tert-butyl dicarbonatewith water;sodium hydroxide in tetrahydrofuran; for 2 h;
Steps:
20.1 Step 1: tert-Butyl [(4-bromo-2-furyl)methyl] carbamate
Step 1: tert-Butyl [(4-bromo-2-furyl)methyl] carbamate [00273] 4-Bromo-2-furaldehyde [Aldrich, product 666599] (10.0 g, 57.1 mmol) was dissolved in ethanol (50 mL) and water (50 mL). N-Hydroxyamine hydrochloride (7.15 g, 103 mmol) and sodium acetate (8.44 g, 103 mmol) were added sequentially and the reaction mixture was brought to reflux at 100 °C for 1 hour. The solution was partially concentrated and the precipitate was collected and washed with cold water (2 x 10 mL). The filtrate was extracted with ethyl acetate (3 x 25 mL) and the combined organic layers were washed with brine (50 mL). After drying over sodium sulfate, the solution was concentrated in vacuo. The residue was combined with the precipitate and dissolved in acetic acid (70 mL). After placing in an ice-bath, zinc (14.7 g, 225 mmol) was added portion-wise over 25 minutes. The reaction warmed to room temperature over 1.5 hours and was filtered through C elite. The solvent was removed in vacuo. [00274] The residue was stirred in tetrahydrofuran (72 mL). A solution of 2.0 Ν NaOH in water (179 mL, 358 mmol) was added dropwise over 45 minutes. After 5 minutes, di-fert-butyldicarbonate (16.9 g, 77.4 mmol) was added dropwise. The reaction was stirred for 2 hours and the tetrahydrofuran was removed in vacuo. Ethyl acetate (100 mL) was added and the suspension was filtered. The organic layer was collected and the product extracted with ethyl acetate (2 x 50 mL). The combined organic layers were washed with brine (100 mL) and water (100 mL), dried over sodium sulfate and concentrated in vacuo to give the desired product (15.3 g, 79%). LCMS calculated for Ci0Hi4BrNNaO3 (M+Na)+: m/z = 298.0. H NMR (400 MHz, DMSO-d6): δ 7.79 (s, 1 H), 7.37 (t, J = 5.8 Hz, 1 H), 6.33 (s, 1 H), 4.06 (d, J= 6.1 Hz, 2 H), 1.36 (s, 9 H).
References:
WO2014/66834,2014,A1 Location in patent:Paragraph 00273-00274

21921-76-6
155 suppliers
$15.00/250mg

24424-99-5
871 suppliers
$13.50/25G
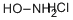
5470-11-1
816 suppliers
$10.00/5G
![Carbamic acid,N-[(4-bromo-2-furanyl)methyl]-, 1,1-dimethylethyl ester](/CAS/20180703/GIF/1204655-02-6.gif)
1204655-02-6
3 suppliers
inquiry