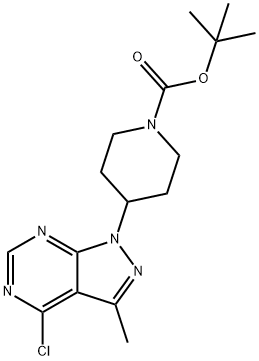
TERT-BUTYL 4-(4-CHLORO-3-METHYL-1H-PYRAZOLO[3,4-D]PYRIMIDIN-1-YL)PIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 4-(4-CHLORO-3-METHYL-1H-PYRAZOLO[3,4-D]PYRIMIDIN-1-YL)PIPERIDINE-1-CARBOXYLATE
- CAS Number:1205537-34-3
- Molecular formula:C16H22ClN5O2
- Molecular Weight:351.83
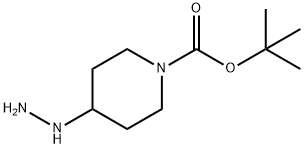
1196486-69-7
19 suppliers
inquiry

60025-06-1
78 suppliers
$13.00/100mg
![TERT-BUTYL 4-(4-CHLORO-3-METHYL-1H-PYRAZOLO[3,4-D]PYRIMIDIN-1-YL)PIPERIDINE-1-CARBOXYLATE](/CAS/20180528/GIF/1205537-34-3.gif)
1205537-34-3
5 suppliers
inquiry
Yield:1205537-34-3 12%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20 - 60; for 21.5 h;Inert atmosphere;
Steps:
13.2
Step 2 - Synthesis of Compound 13B; Compound 13A (860 mg) was dissolved in DMF (5 mL) and to the resulting solution was added DIPEA (1.1 mL, 1.4 eq), followed by compound 12B (1.36 g, 1.4 eq). The resulting reaction was allowed to stir at room temperature under nitrogen atmosphere for 2.5 hours, then the reaction mixture was heated at 60 0C and allowed to stir at this temperature for an additional 19 hours. The reaction mixture was cooled to room temperature and concentrated in vacuo to provide a brown oily residue which was purified using flash column chromatography on silica gel to provide compound 13B (192 mg, 12%).
References:
WO2010/9207,2010,A1 Location in patent:Page/Page column 58