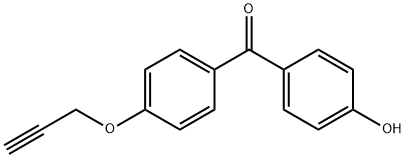
(4-Hydroxyphenyl)(4-(prop-2-yn-1-yloxy)phenyl)methanone synthesis
- Product Name:(4-Hydroxyphenyl)(4-(prop-2-yn-1-yloxy)phenyl)methanone
- CAS Number:1208395-99-6
- Molecular formula:C16H12O3
- Molecular Weight:252.26
611-99-4
427 suppliers
$6.00/5g

106-96-7
332 suppliers
$31.00/25g

1208395-99-6
33 suppliers
$85.00/5mg
Yield:1208395-99-6 82%
Reaction Conditions:
with potassium carbonate in acetone at 0 - 50; for 3 h;
Steps:
(4-hydroxyphenyl)(4-(prop-2-yn-1-yloxy)phenyl)methanone (6')
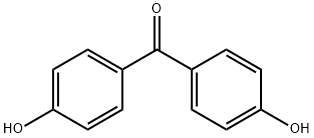
To a solution of 4,4'-dihydroxybenzophone (500 mg,2.33 mmol) in acetone (5.0 ml) at 0°C, K2CO3 (161 mg, 1.17 mmol) andpropargyl bromide (0.11 ml, 1.16 mmol) were added. The reaction mixture wasstirred at 50 °C for 3 h, cooled to room temperature, and quenched with H2O (10 mL). The mixture was extracted threetimes with ethyl acetate. The combined extracts were washed with brine, dried over MgSO4, filtered, andconcentrated. The residue was purified via Biotage (ethyl acetate / n-hexane = 1 : 2 Snap Ultra25g column). The desired fractions were collected and concentrated togive propargylatedbenzophenone (6') (240 mg, 82% BRSM). 1H-NMR (600 MHz, CD3OD)δ 7.73 (d, 2H, J = 8.4 Hz), 7.67 (d,2H, J = 8.4 Hz), 7.09 (d, 2H, J = 9.0 Hz), 6.87 (d, 2H, J = 9.0 Hz), 4.82 (s, 2H), 3.00 (t , 1H,J = 2.4 Hz); 13C-NMR (150MHz, CD3OD) δ 195.4, 162.0, 161.0, 132.3, 131.6, 131.1, 128.9,114.7, 114.7, 77.8, 75.9, 55.3; HRMS(ESI): mass calcd for C16H15O3 [M + H] +, 253.0786; found, 253.0872.
References:
Lee, Bit;Sun, Wei;Lee, Hyungjun;Basavarajappa, Halesha;Sulaiman, Rania S.;Sishtla, Kamakshi;Fei, Xiang;Corson, Timothy W.;Seo, Seung-Yong [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 17,p. 4277 - 4281] Location in patent:supporting information