
(R)-TERT-BUTYL 3-(2-METHOXYETHOXY) PYRROLIDINE-1-CARBOXYLATE synthesis
- Product Name:(R)-TERT-BUTYL 3-(2-METHOXYETHOXY) PYRROLIDINE-1-CARBOXYLATE
- CAS Number:1212413-55-2
- Molecular formula:C12H23NO4
- Molecular Weight:245.32
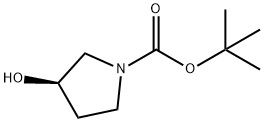
109431-87-0
356 suppliers
$5.00/1g

6482-24-2
285 suppliers
$9.00/1g

1212413-55-2
7 suppliers
inquiry
Yield:1212413-55-2 51%
Reaction Conditions:
Stage #1: (R)-tert-butyl 3-hydroxypyrrolidine-1-carboxylatewith sodium hydride in tetrahydrofuran;mineral oil at 0; for 0.5 h;Inert atmosphere;
Stage #2: 2-Bromoethyl methyl ether in tetrahydrofuran;mineral oil at 20;
Steps:
2 Synthesis of tert-butyl (R)-3-(2-methoxyethoxy)pyrrolidine-1-carboxylate:
To a stirred solution of NaH (3.8 g, 95 mmol, 60% in mineral oil w/w) in THF (20 ml) under N2atmosphere at 0 °C, was added tert-butyl (R)-3-hydroxypyrrolidine-l- carboxylate (step 1, 4.5 g, 24 mmol) in THF (40 ml). After 30 minutes l-bromo-2- methoxyethane (3.4 ml, 36 mmol) was added, the reaction mixture was slowly allowed to attain to room temperature and stirred for overnight. After completion of the reaction (monitored by TLC), the reaction mixture was quenched by addition of saturated NH4C1 solution at 0 °C. The solution was extracted with EtOAc (2x100 mL), the combined organic phases were washed with brine, dried over Na2S04and concentrated under reduced pressure to give the crude product. Purification by flash chromatography with EtOAc-hexane (2: 8) as an eluent to afford the desired product (3.0 g, yield: 51%) as an oil. 1H MR (300 MHz, CDC13): δ 4.10-4.05 (m, 1H), 3.60-3.52 (m, 4H), 3.45-3.36 (m, 4H), 3.38 (s, 3H), 2.02-1.93 (m, 2H), 1.45 (s, 9H).
References:
WO2017/17630,2017,A1 Location in patent:Page/Page column 36

24424-99-5
824 suppliers
$13.50/25G

1212413-55-2
7 suppliers
inquiry