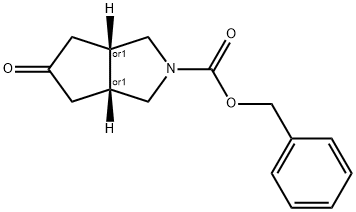
(3aR,6aS)-benzyl 5-oxohexahydrocyclopenta[c]pyrrole-2(1H)-carboxylate synthesis
- Product Name:(3aR,6aS)-benzyl 5-oxohexahydrocyclopenta[c]pyrrole-2(1H)-carboxylate
- CAS Number:1217315-21-3
- Molecular formula:C15H17NO3
- Molecular Weight:259.3
Yield:1217315-21-3 73%
Reaction Conditions:
in 1,2-dimethoxyethane;hexane;water at 0 - 100; for 3 h;
Steps:
1.3 Step-3: Preparation of benzyl 5-oxohexahydrocyclopenta[c]pyrrole-2(lH)-carboxylate
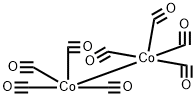
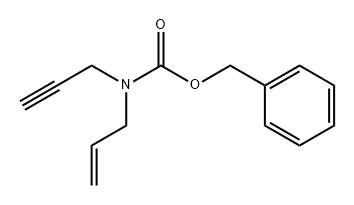
Step-3: Preparation of benzyl 5-oxohexahydrocyclopenta[c]pyrrole-2(lH)-carboxylate H Cbz-N^ ^=0 H [0118] To a stirred solution of benzyl allyl(prop-2-yn-l-yl)carbamate (40 g, 174.4 mmol) in a mixture of water and dimethoxy ethane (65 mL: 400 mL) was added dicobalt octacarbonyl [moistened with hexane (hexane 1-10%), > 90% (Co)] (72 g, 191.9 mmol) at 0 °C. The reaction mixture was allowed to warm to room temperature slowly and heated to 100 °C and stirred for 3 h. After completion of reaction (monitored by TLC), the reaction mixture was concentrated to afford crude product which was purified by silica gel column chromatography (50% ethylacetate/hexane) to obtain the title compound benzyl 5- oxohexahydrocyclopenta[c]pyrrole-2(lH)-carboxylate (33 g, 73 % yield) as a brown solid. Calculated (M+H): 260.12; Found (M+H): 260.2.
References:
WO2015/48507,2015,A1 Location in patent:Paragraph 0118
![hexahydrocyclopenta[c]pyrrol-5(1H)-one](/CAS/GIF/96896-09-2.gif)
96896-09-2
28 suppliers
$156.00/50mg

501-53-1
405 suppliers
$10.00/1g
![(3aR,6aS)-benzyl 5-oxohexahydrocyclopenta[c]pyrrole-2(1H)-carboxylate](/CAS/20150408/GIF/1217315-21-3.gif)
1217315-21-3
11 suppliers
inquiry

2144-87-8
13 suppliers
$98.00/50mg
![(3aR,6aS)-benzyl 5-oxohexahydrocyclopenta[c]pyrrole-2(1H)-carboxylate](/CAS/20150408/GIF/1217315-21-3.gif)
1217315-21-3
11 suppliers
inquiry
5041-33-8
30 suppliers
$90.00/250mg
![(3aR,6aS)-benzyl 5-oxohexahydrocyclopenta[c]pyrrole-2(1H)-carboxylate](/CAS/20150408/GIF/1217315-21-3.gif)
1217315-21-3
11 suppliers
inquiry

501-53-1
405 suppliers
$10.00/1g
![(3aR,6aS)-benzyl 5-oxohexahydrocyclopenta[c]pyrrole-2(1H)-carboxylate](/CAS/20150408/GIF/1217315-21-3.gif)
1217315-21-3
11 suppliers
inquiry