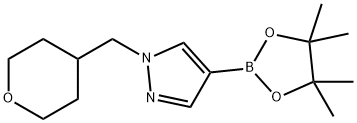
1-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole synthesis
- Product Name:1-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
- CAS Number:1220635-60-8
- Molecular formula:C15H25BN2O3
- Molecular Weight:292.18

269410-08-4
389 suppliers
$5.00/1g
101691-94-5
112 suppliers
$22.00/0.25/ G
![1-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole](/CAS/GIF/1220635-60-8.gif)
1220635-60-8
29 suppliers
$45.00/10mg
Yield:1220635-60-8 3.8%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 80; for 5 h;
Steps:
Synthesis of boronic ester R7.6:
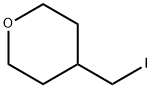
Synthesis of boronic ester R7.6: 2 g (10.3 mmol) 4-(4,4,5,5-Tetramethyl-l,3,2-dioxaborolan-2-yl)-lH-pyrazole and 2.9 mL (20.6 mmol) 4-(iodomethyl)-tetrahydro-2H-pyran are dissolved in 200 mL DMF and 4.274 g (30.9 mmol) K2CO3 are added. The mixture is shaken at 80°C for 5 h. After cooling to r.t. the mixture is filtered, the filtrate is concentrated in vacuo to approximately 60 mL. The product is separated using HPLC-MS (Gilson, mass flow 120 mL/min, 10 μιη, 200g Sunfire RP18, ACN/water/TFA). The product fractions are combined and freeze-dried to yield 115 mg product (3.8 %) R7.6.
References:
WO2014/140075,2014,A1 Location in patent:Page/Page column 219-220