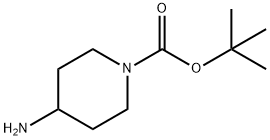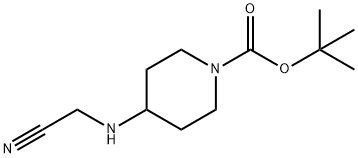
tert-Butyl 4-((cyanoMethyl)aMino)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-((cyanoMethyl)aMino)piperidine-1-carboxylate
- CAS Number:1224923-48-1
- Molecular formula:C12H21N3O2
- Molecular Weight:239.31
Yield:1224923-48-1 295 mg
Reaction Conditions:
with potassium carbonate in acetonitrile at 80; for 23 h;
Steps:
Intermediate 7 (INT 7)
A mixture of tert-butyl 4-aminopiperidine-1-carboxylate (303 mg, 1.51 mmol) , 2-chloroacetonitrile (121 mg, 1.60 mmol) and K 2CO 3 (420 mg, 3.04 mmol) in ACN (10 mL) was heated to 80 for 23 h. The mixture was filtered and concentrated. The residue was purified by silica gel chromatography (eluting with 0-35 %DCM /MeOH) to provide INT 7 (295 mg) . MS m/z 240 [M+H] +.
References:
WO2022/105855,2022,A1 Location in patent:Page/Page column 152
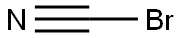
506-68-3
148 suppliers
$10.00/1g
147539-41-1
216 suppliers
$6.00/1g

1224923-48-1
4 suppliers
inquiry